Key Nutrition for Thyroid Disease | Wellness Clinic
The thyroid gland is a 2-inch butterfly-shaped organ located in the front part of the neck. Although small, the thyroid glans is a major gland in the endocrine system and affects virtually every organ in the body.
What is the function of the thyroid gland?
The thyroid gland regulates fat and carbohydrate metabolism, respiration, body temperature, brain growth, cholesterol levels, the heart and nervous system, blood glucose levels cycle, skin integrity, and more.
Thyroid Diseases Explained
Thyroid disease generally involves an underactive thyroid gland, also known as hypothyroidism. In the USA, an autoimmune reaction called autoimmune thyroiditis or Hashimoto’s disease usually causes hypothyroidism. As with all autoimmune disorders, the body identifies its own tissues as an invader and strikes until the organ is destroyed. This chronic attack will finally prevent the thyroid gland from producing thyroid hormones. The lack of these hormones may slow down metabolism and also cause weight gain, fatigue, dry skin and hair loss as well as lead to difficulty concentrating. Hashimoto’s thyroid disease affects approximately 5 percent of the US population, is seven times more prevalent in women than men, and generally occurs during middle age.
Hyperthyroidism, or an overactive thyroid gland, is another frequent thyroid disease. The form is Graves’ disease in which the body’s autoimmune reaction causes the thyroid gland to make too much T3 and T4. Symptoms of hyperthyroidism may include weight loss, high blood pressure, nausea, and a rapid heartbeat. The disease also disproportionately affects women and presents until the age of 40.
Hashimoto’s thyroid disease is more common than Graves’ disease, but both are known as autoimmune thyroid disease (ATD), which has a strong genetic link and is associated with other autoimmune disorders, such as type 1 diabetes, rheumatoid arthritis, lupus, and celiac disease. A goiter, or enlargement of the thyroid gland, may be caused by hypothyroidism, hyperthyroidism, excessive or insufficient consumption of iodine from the diet, or thyroid gland, the most frequent endocrine cancer whose prevalence studies imply is increasing.
Key Nutrients for Thyroid Disease
Many dietary factors play a role in optimizing thyroid function. But, excesses and both nutrient deficiencies could cause or exacerbate symptoms. Working in collaboration with a doctor is ideal to determine status for optimal thyroid health. Many functional medicine practitioners specialize in functional nutrition, which can help with thyroid disease.
Iodine
Iodine is a vital nutrient in the human body and essential to thyroid function; thyroid hormones have been constituted of iodine. Iodine deficiency is the cause while disorder is the primary cause of thyroid dysfunction in the United States
Iodine deficiency has been considered uncommon in america since the 1920s, largely as a result of widespread utilization of iodized salt. This, along with poultry, milk, and grains, is a major source of iodine in the conventional American diet.
However, iodine intake has decreased during the last few decades. Americans get approximately 70 percent of their salt intake from foods which, in the USA and Canada, don’t contain iodine. A 2012 Centers for Disease Control and Prevention report indicates that, on average, Americans are receiving sufficient amounts of iodine, together with the potential exclusion of women of childbearing age.
Both iodine deficiency and surplus have significant dangers; thus, supplementation ought to be approached with care. Supplemental iodine might lead to symptom flare-ups in individuals with Hashimoto’s thyroid disease because it stimulates antibodies.
Iodine intake often is not easily apparent on a dietary recall because the quantity in foods is largely determined by levels from the soil and extra salt. But, experts state that, “Clients carrying iodine tablets are a red flag. Frequent intake of foods such as seaweed or an avoidance of all iodized salt may serve as signals that further exploration is required.”
Vitamin D
Vitamin D deficiency is connected to Hashimoto’s, according to one study showing that over 90 percent of patients studied were deficient. It’s uncertain whether the low vitamin D levels were the direct cause of Hashimoto’s or the result of the disease process itself.
Hyperthyroidism, especially Graves’ disease, is known to cause bone loss, which can be compounded by the vitamin D deficiency commonly seen in people with hyperthyroidism. This bone mass could be recovered with therapy for hyperthyroidism, and specialists indicate that sufficient nourishment, such as vitamin D, which are particularly important during and following
Foods which contain some vitamin D include fatty fish, milk, legumes, eggs, and mushrooms. Sunlight also is a source, but the sum of vitamin production depends upon the season and latitude. Supplemental D3 could be necessary, if clients have low vitamin D levels, along with the customer’s doctor should monitor progress to ensure the individual’s levels stay within a suitable range.
Selenium
The maximum concentration of selenium is found in the thyroid gland, and it has been demonstrated to be a necessary element of enzymes integral to thyroid function. Selenium is a vital trace mineral and was shown to have a deep effect in the immune system, cognitive function, fertility in both women and men, and mortality rate.
A meta-analysis of randomized, placebo-controlled studies has shown advantages of selenium on both the thyroid antibody titers and mood in patients with Hashimoto’s, but this impact appears more pronounced in people who have a selenium deficiency or insufficiency in the outset. Conversely, an excessive intake of selenium can lead to gastrointestinal distress or perhaps raise the risk of type 2 diabetes and cancer. So clients will benefit from getting their selenium levels tested and integrating healthful foods into their diets, including Brazil nuts, tuna, crab, and lobster.
Vitamin B12
Studies show that about 30 percent of people with ATD experience a vitamin B12 deficiency. Food sources of B12 include salmon, sardines, mollusks, organ meats such as liver, muscle meat, and dairy. Vegan sources include fortified cereals and yeast. Severe B12 deficiency may be irreversible, therefore it is important for dietitians to suggest clients have their levels analyzed.
Goitrogens
Cruciferous vegetables like broccoli, cauliflower, and cabbage naturally discharge a chemical known as goitrin when they are hydrolyzed, or broken down. Goitrin can interfere with the synthesis of thyroid hormones. Nonetheless, this is usually a concern only when combined with an iodine deficiency. Heating cruciferous vegetables denatures much or all of this possible goitrogenic effect.
Soy is another possible goitrogen. The isoflavones in soy may lower thyroid hormone synthesis, but many studies have discovered that consuming soy does not result in hypothyroidism in individuals with adequate iodine stores. But Dean cautions clients to consume soy in moderation.
The potential exclusion is millet, a nutritious gluten-free grain, which might suppress thyroid function even in people with adequate iodine intake. If a dietary recall indicates frequent millet ingestion in patients with hypothyroidism, it may be wise to indicate they choose another grain.
Foods, Supplements, and Medication Interactions
When it comes to thyroid medications, it is very important to RDs to know the drugs can interact with common nutritional supplements. Calcium supplements have the capacity to interfere with absorption of thyroid medications, so when taking the two patients need to consider the timing. Studies recommend limiting calcium supplements and thyroid drugs by at least four hours. Coffee and fiber nutritional supplements reduced the absorption of thyroid drugs, so patients should take them one hour apart. Dietitians should affirm whether customers have received and are adhering to these guidelines for optimum wellness.
Chromium picolinate, which is marketed for blood sugar control and weight reduction, also impairs the absorption of thyroid medications. If clients decide to take chromium picolinate, then they ought to take it three to four hours apart from thyroid drugs. Flavonoids in vegetables, fruits, and tea have been shown to have potential cardiovascular benefits. But, high-dose flavonoid supplements can suppress thyroid function. The Natural Standards Database provides a comprehensive list of nutritional supplements with a possible impact on thyroid function, thus taking precautions and coordinating patient care with a knowledgeable practitioner is sensible.
Exercise
A discussion on thyroid disorder and good health is not complete without stressing the importance of physical activity. Lisa Lilienfield, MD, a thyroid disorder specialist in the Kaplan Center for Integrative Medicine in McLean, Virginia, and a certified yoga teacher, is a firm believer in the value of exercise, especially. “With hypothyroid patients, certainly exercise can assist with weight gain, fatigue, and depression. With hyperthyroidism, anxiety and sleep disturbances are so common, and exercise might help regulate both.”
In addition to the obvious impact exercise has on weight and metabolism, a study of patients with Graves’ disease found that a structured exercise plan revealed remarkable improvements in fatigue levels, and significantly more patients have been able to successfully quit taking antithyroid medications with no relapse.
In Conclusion
Celiac disease presents unique challenges as a result of unwanted weight changes, significant cardiovascular disease, and symptoms such as fatigue, mood changes, and gastrointestinal upset, which can hinder the growth of healthful behaviors. It’s vital that dietitians focus when counselling clients on setting goals that are realistic for adjustments and routine exercise. With so many nutrient deficiencies and interactions with medications and nutritional supplements, it will be important for dietitians to coordinate with their clients’ health care team for health outcomes.
The scope of our information is limited to chiropractic and spinal injuries and conditions. To discuss options on the subject matter, please feel free to ask Dr. Jimenez or contact us at 915-850-0900 .�
By Dr. Alex Jimenez
Additional Topics: Wellness
Overall health and wellness are essential towards maintaining the proper mental and physical balance in the body. From eating a balanced nutrition as well as exercising and participating in physical activities, to sleeping a healthy amount of time on a regular basis, following the best health and wellness tips can ultimately help maintain overall well-being. Eating plenty of fruits and vegetables can go a long way towards helping people become healthy.

TRENDING TOPIC: EXTRA EXTRA: About Chiropractic


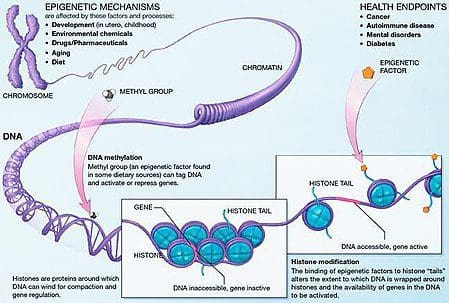 Obesity is a complex, multifactorial disease, and better understanding of the mechanisms underlying the interactions between lifestyle, environment, and genetics is critical for developing effective strategies for prevention and treatment [1].
Obesity is a complex, multifactorial disease, and better understanding of the mechanisms underlying the interactions between lifestyle, environment, and genetics is critical for developing effective strategies for prevention and treatment [1]. Animal models provide unique opportunities for highly controlled studies that provide mechanistic insight into�the role of specific epigenetic marks, both as indicators of current metabolic status and as predictors of the future risk of obesity and metabolic disease. A particularly important aspect of animal studies is that they allow for the assessment of epigenetic changes within target tissues, including the liver and hypothalamus, which is much more difficult in humans. Moreover, the ability to harvest large quantities of fresh tissue makes it possible to assess multiple chromatin marks as well as DNA methylation. Some of these epigenetic modifications either alone or in combination may be responsive to environmental programming. In animal models, it is also possible to study multiple generations of offspring and thus enable differentiation between trans-generational and intergenerational transmission of obesity risk mediated by epigenetic memory of parental nutritional status, which cannot be easily distinguished in human studies. We use the former term for meiotic transmission of risk in the absence of continued exposure while the latter primarily entails direct transmission of risk through metabolic reprogramming of the fetus or gametes.
Animal models provide unique opportunities for highly controlled studies that provide mechanistic insight into�the role of specific epigenetic marks, both as indicators of current metabolic status and as predictors of the future risk of obesity and metabolic disease. A particularly important aspect of animal studies is that they allow for the assessment of epigenetic changes within target tissues, including the liver and hypothalamus, which is much more difficult in humans. Moreover, the ability to harvest large quantities of fresh tissue makes it possible to assess multiple chromatin marks as well as DNA methylation. Some of these epigenetic modifications either alone or in combination may be responsive to environmental programming. In animal models, it is also possible to study multiple generations of offspring and thus enable differentiation between trans-generational and intergenerational transmission of obesity risk mediated by epigenetic memory of parental nutritional status, which cannot be easily distinguished in human studies. We use the former term for meiotic transmission of risk in the absence of continued exposure while the latter primarily entails direct transmission of risk through metabolic reprogramming of the fetus or gametes.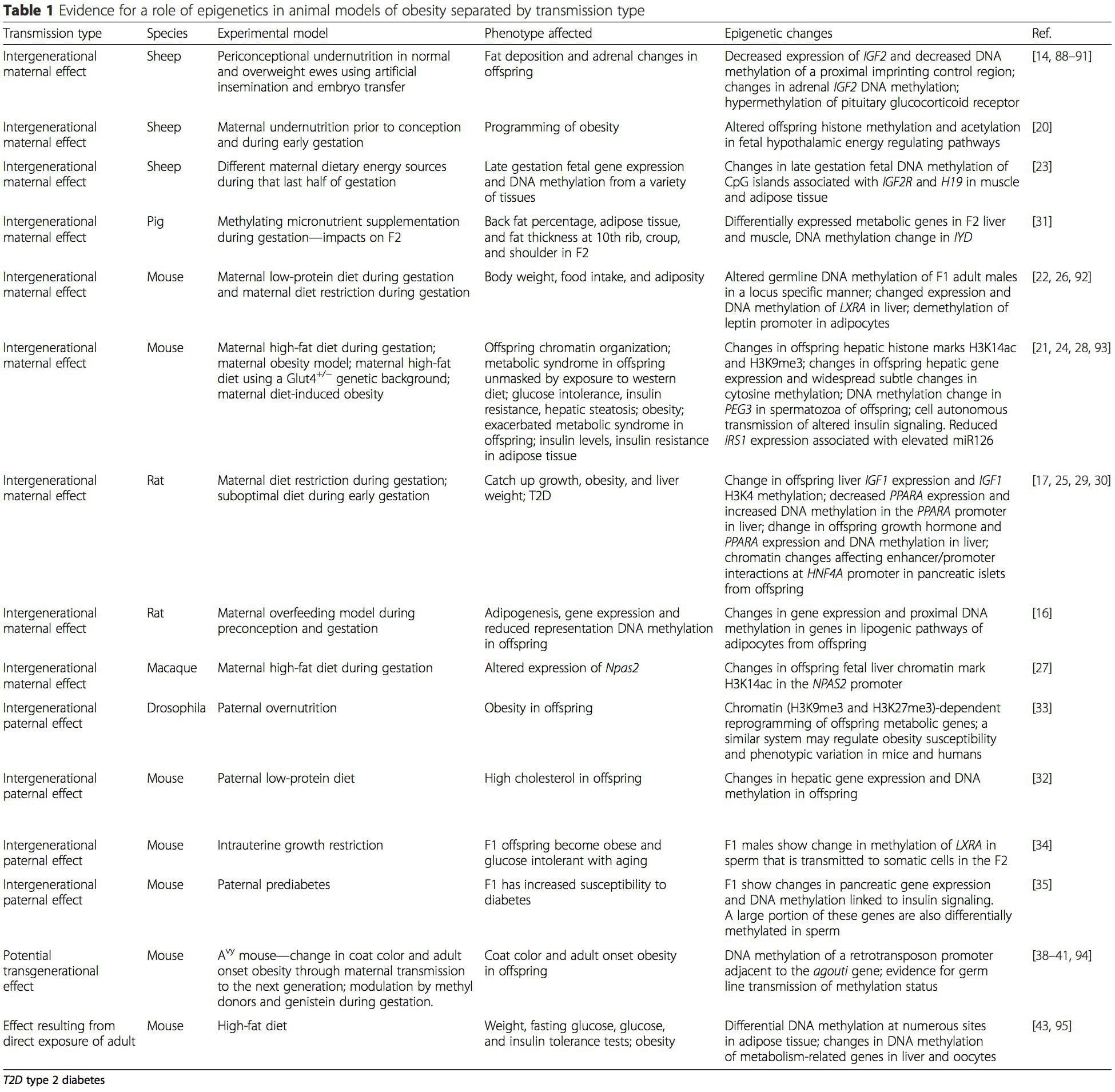 (i) Epigenetic Changes In Offspring Associated With Maternal Nutrition During Gestation
(i) Epigenetic Changes In Offspring Associated With Maternal Nutrition During Gestation Maternal nutritional supplementation, undernutrition, and over nutrition during pregnancy can alter fat deposition and energy homeostasis in offspring [11, 13�15, 19]. Associated with these effects in the offspring are changes in DNA methylation, histone post-translational modifications, and gene expression for several target genes,�especially genes regulating fatty acid metabolism and insulin signaling [16, 17, 20�30]. The diversity of animal models used in these studies and the common metabolic pathways impacted suggest an evolutionarily conserved adaptive response mediated by epigenetic modification. However, few of the specific identified genes and epigenetic changes have been cross-validated in related studies, and large-scale genome-wide investigations have typically not been applied. A major hindrance to comparison of these studies is the different develop mental windows subjected to nutritional challenge, which may cause considerably different outcomes. Proof that the epigenetic changes are causal rather than being associated with offspring phenotypic changes is also required. This will necessitate the identification of a parental nutritionally induced epigenetic �memory� response that precedes development of the altered phenotype in offspring.
Maternal nutritional supplementation, undernutrition, and over nutrition during pregnancy can alter fat deposition and energy homeostasis in offspring [11, 13�15, 19]. Associated with these effects in the offspring are changes in DNA methylation, histone post-translational modifications, and gene expression for several target genes,�especially genes regulating fatty acid metabolism and insulin signaling [16, 17, 20�30]. The diversity of animal models used in these studies and the common metabolic pathways impacted suggest an evolutionarily conserved adaptive response mediated by epigenetic modification. However, few of the specific identified genes and epigenetic changes have been cross-validated in related studies, and large-scale genome-wide investigations have typically not been applied. A major hindrance to comparison of these studies is the different develop mental windows subjected to nutritional challenge, which may cause considerably different outcomes. Proof that the epigenetic changes are causal rather than being associated with offspring phenotypic changes is also required. This will necessitate the identification of a parental nutritionally induced epigenetic �memory� response that precedes development of the altered phenotype in offspring. Emerging studies have demonstrated that paternal plane of nutrition can impact offspring fat deposition and epigenetic marks [31�34]. One recent investigation using mice has demonstrated that paternal pre-diabetes leads to increased susceptibility to diabetes in F1 offspring with associated changes in pancreatic gene expression and DNA methylation linked to insulin signaling [35]. Importantly, there was an overlap of these epigenetic changes in pancreatic islets and sperm suggesting germ line inheritance. However, most of these studies, although intriguing in their implications, are limited in the genomic scale of investigation and frequently show weak and somewhat transient epigenetic alterations associated with mild metabolic phenotypes in offspring.
Emerging studies have demonstrated that paternal plane of nutrition can impact offspring fat deposition and epigenetic marks [31�34]. One recent investigation using mice has demonstrated that paternal pre-diabetes leads to increased susceptibility to diabetes in F1 offspring with associated changes in pancreatic gene expression and DNA methylation linked to insulin signaling [35]. Importantly, there was an overlap of these epigenetic changes in pancreatic islets and sperm suggesting germ line inheritance. However, most of these studies, although intriguing in their implications, are limited in the genomic scale of investigation and frequently show weak and somewhat transient epigenetic alterations associated with mild metabolic phenotypes in offspring.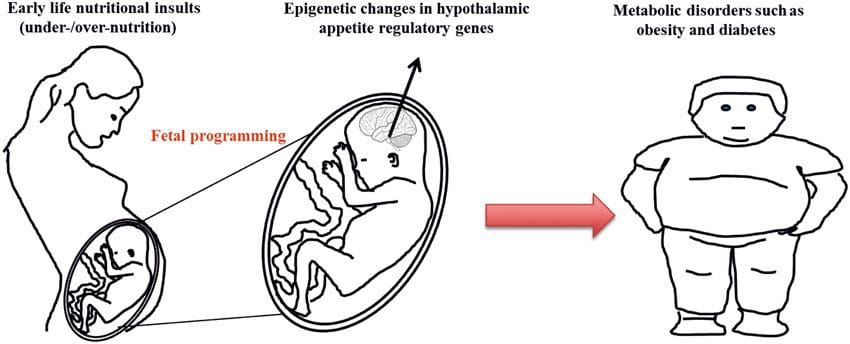 Stable transmission of epigenetic information across multiple generations is well described in plant systems and C. elegans, but its significance in mammals is still much debated [36, 37]. An epigenetic basis for grand- parental transmission of phenotypes in response to dietary exposures has been well established, including in livestock species [31]. The most influential studies demonstrating effects of epigenetic transmission impacting offspring phenotype have used the example of the viable yellow agouti (Avy) mouse [38]. In this mouse, an insertion of a retrotransposon upstream of the agouti gene causes its constitutive expression and consequent yellow coat color and adult onset obesity. Maternal transmission through the germ line results in DNA methylation�mediated silencing of agouti expression resulting in wild-type coat color and lean phenotype of the offspring [39, 40]. Importantly, subsequent studies in these mice demonstrated that maternal exposure to methyl donors causes a shift in coat color [41]. One study has reported transmission of a phenotype to the F3 generation and alterations in expression of large number of genes in response to protein restriction in F0 [42]; however, alterations in expression were highly variable and a direct link to epigenetic changes was not identified in this system.
Stable transmission of epigenetic information across multiple generations is well described in plant systems and C. elegans, but its significance in mammals is still much debated [36, 37]. An epigenetic basis for grand- parental transmission of phenotypes in response to dietary exposures has been well established, including in livestock species [31]. The most influential studies demonstrating effects of epigenetic transmission impacting offspring phenotype have used the example of the viable yellow agouti (Avy) mouse [38]. In this mouse, an insertion of a retrotransposon upstream of the agouti gene causes its constitutive expression and consequent yellow coat color and adult onset obesity. Maternal transmission through the germ line results in DNA methylation�mediated silencing of agouti expression resulting in wild-type coat color and lean phenotype of the offspring [39, 40]. Importantly, subsequent studies in these mice demonstrated that maternal exposure to methyl donors causes a shift in coat color [41]. One study has reported transmission of a phenotype to the F3 generation and alterations in expression of large number of genes in response to protein restriction in F0 [42]; however, alterations in expression were highly variable and a direct link to epigenetic changes was not identified in this system.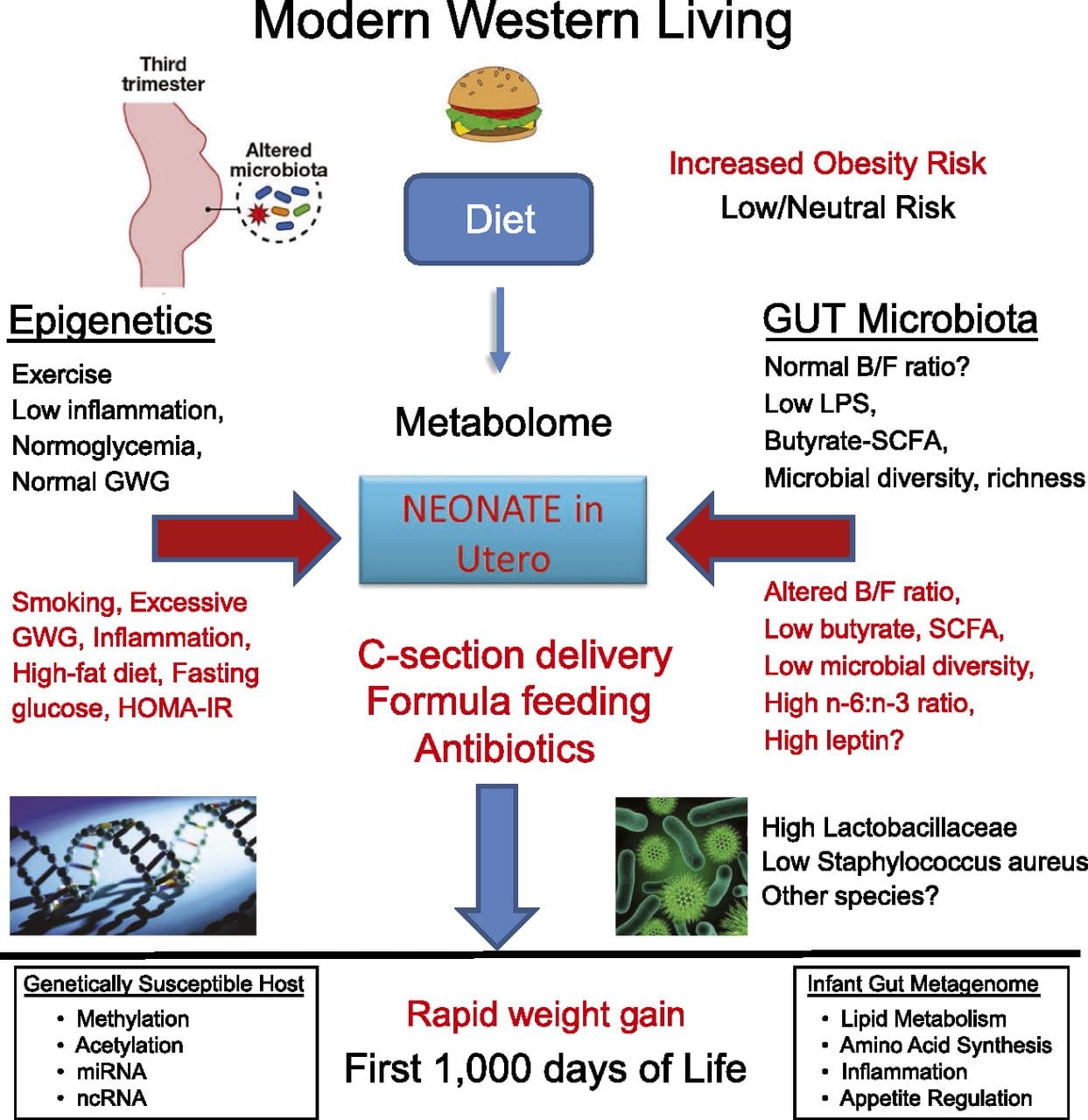 While many studies have identified diet-associated epigenetic changes in animal models using candidate site-specific regions, there have been few genome-wide analyses undertaken. A recent study focussed on determining the direct epigenetic impact of high-fat diets/ diet-induced obesity in adult mice using genome-wide gene expression and DNA methylation analyses [43]. This study identified 232 differentially methylated regions (DMRs) in adipocytes from control and high-fat fed mice. Importantly, the corresponding human regions for the murine DMRs were also differentially methylated in adipose tissue from a population of obese and lean humans, thereby highlighting the remarkable evolutionary conservation of these regions. This result emphasizes the likely importance of the identified DMRs in regulating energy homeostasis in mammals.
While many studies have identified diet-associated epigenetic changes in animal models using candidate site-specific regions, there have been few genome-wide analyses undertaken. A recent study focussed on determining the direct epigenetic impact of high-fat diets/ diet-induced obesity in adult mice using genome-wide gene expression and DNA methylation analyses [43]. This study identified 232 differentially methylated regions (DMRs) in adipocytes from control and high-fat fed mice. Importantly, the corresponding human regions for the murine DMRs were also differentially methylated in adipose tissue from a population of obese and lean humans, thereby highlighting the remarkable evolutionary conservation of these regions. This result emphasizes the likely importance of the identified DMRs in regulating energy homeostasis in mammals.
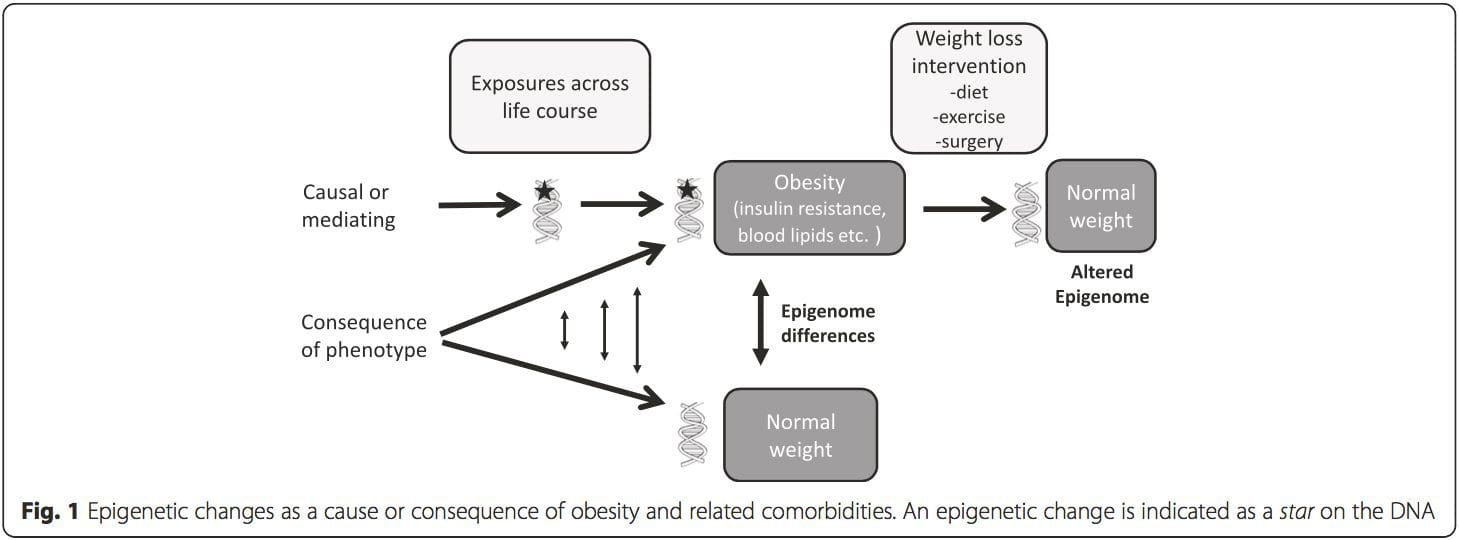 (i) Genetic association studies. Genetic polymorphisms that are associated with an increased risk of developing particular conditions are a priori linked to the causative genes. The presence of differential�methylation in such regions infers functional relevance of these epigenetic changes in controlling expression of the proximal gene(s). There are strong cis-acting genetic effects underpinning much epigenetic variation [7, 45], and in population-based studies, methods that use genetic surrogates to infer a causal or mediating role of epigenome differences have been applied [7, 46�48]. The use of familial genetic information can also lead to the identification of potentially causative candidate regions showing phenotype-related differential methylation [49].
(i) Genetic association studies. Genetic polymorphisms that are associated with an increased risk of developing particular conditions are a priori linked to the causative genes. The presence of differential�methylation in such regions infers functional relevance of these epigenetic changes in controlling expression of the proximal gene(s). There are strong cis-acting genetic effects underpinning much epigenetic variation [7, 45], and in population-based studies, methods that use genetic surrogates to infer a causal or mediating role of epigenome differences have been applied [7, 46�48]. The use of familial genetic information can also lead to the identification of potentially causative candidate regions showing phenotype-related differential methylation [49].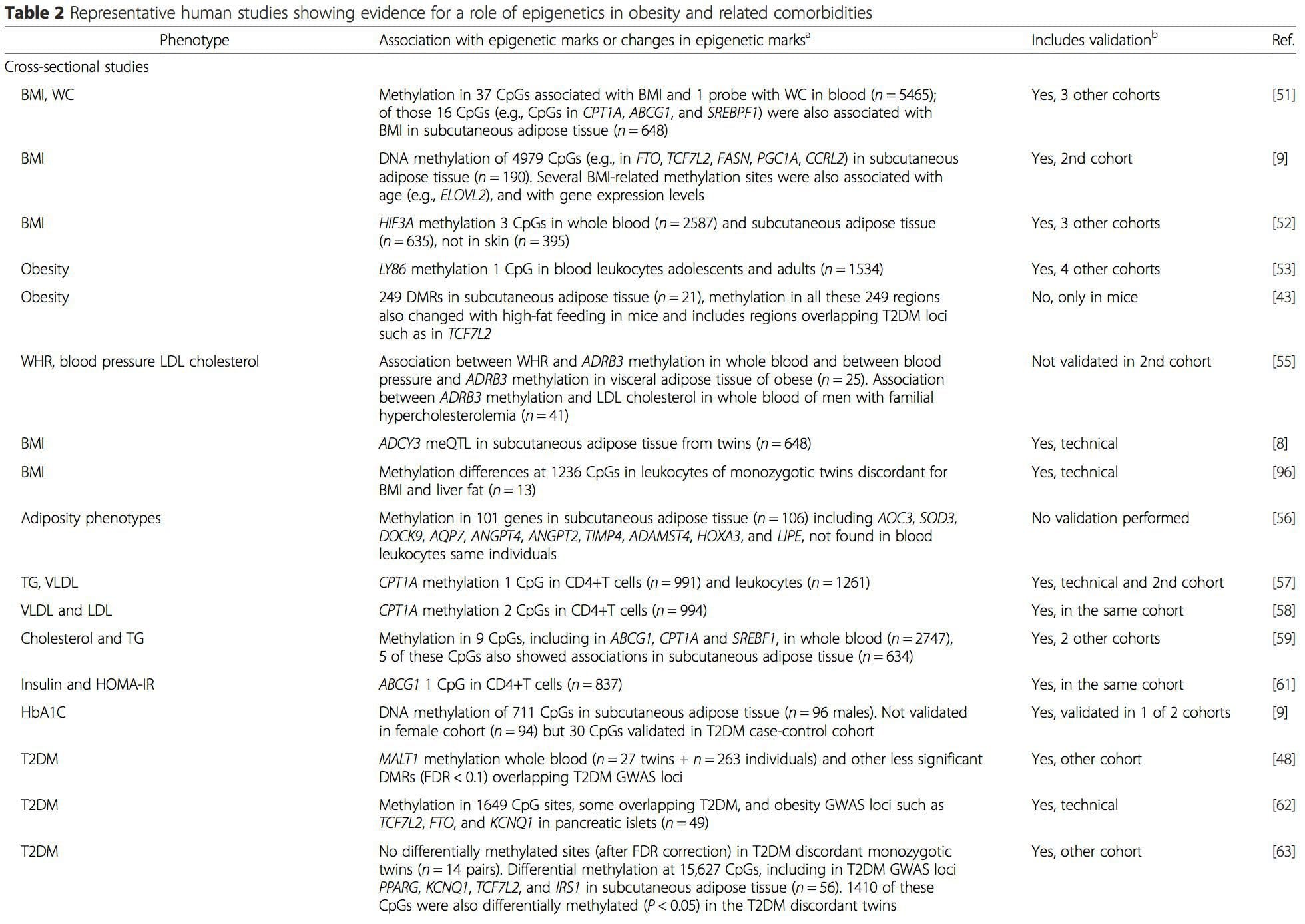
 From these studies, altered methylation of PGC1A, HIF3A, ABCG1, and CPT1A and the previously described RXRA [18] have emerged as biomarkers associated with, or perhaps predictive of, metabolic health that are also plausible candidates for a role in development of metabolic disease.
From these studies, altered methylation of PGC1A, HIF3A, ABCG1, and CPT1A and the previously described RXRA [18] have emerged as biomarkers associated with, or perhaps predictive of, metabolic health that are also plausible candidates for a role in development of metabolic disease.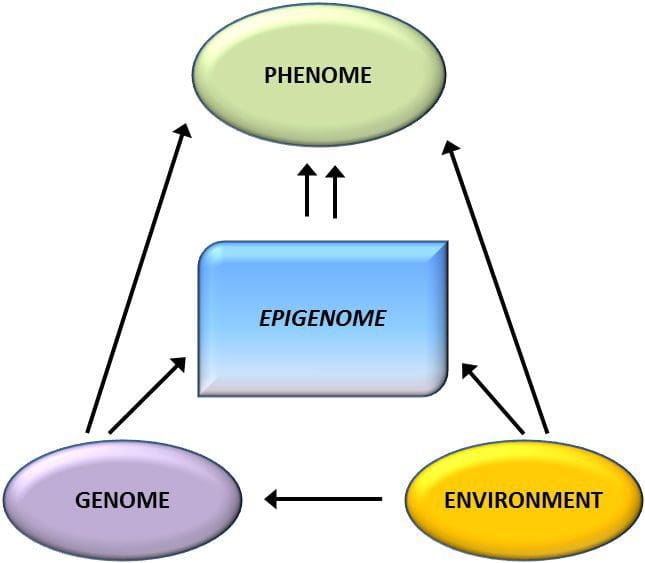 Epigenetic variation is highly influenced by the underlying genetic variation, with genotype estimated to explain ~20�40 % of the variation [6, 8]. Recently, a number of studies have begun to integrate methylome and genotype data to identify methylation quantitative trait loci (meQTL) associated with disease phenotypes. For instance, in adipose tissue, an meQTL overlapping�with a BMI genetic risk locus has been identified in an enhancer element upstream of ADCY3 [8]. Other studies have also identified overlaps between known obesity and T2DM risk loci and DMRs associated with obesity and T2DM [43, 48, 62]. Methylation of a number of such DMRs was also modulated by high-fat feeding in mice [43] and weight loss in humans [64]. These results identify an intriguing link between genetic variations linked with disease susceptibility and their association with regions of the genome that undergo epigenetic modifications in response to nutritional challenges, implying a causal relationship. The close connection between genetic and epigenetic variation may signify their essential roles in generating individual variation [65, 66]. However, while these findings suggest that DNA methylation may be a mediator of genetic effects, it is also important to consider that both genetic and epigenetic processes could act independently on the same genes. Twin studies [8, 63, 67] can provide important insights and indicate that inter-individual differences in levels of DNA methylation arise predominantly from non-shared environment and stochastic influences, minimally from shared environmental effects, but also with a significant impact of genetic variation.
Epigenetic variation is highly influenced by the underlying genetic variation, with genotype estimated to explain ~20�40 % of the variation [6, 8]. Recently, a number of studies have begun to integrate methylome and genotype data to identify methylation quantitative trait loci (meQTL) associated with disease phenotypes. For instance, in adipose tissue, an meQTL overlapping�with a BMI genetic risk locus has been identified in an enhancer element upstream of ADCY3 [8]. Other studies have also identified overlaps between known obesity and T2DM risk loci and DMRs associated with obesity and T2DM [43, 48, 62]. Methylation of a number of such DMRs was also modulated by high-fat feeding in mice [43] and weight loss in humans [64]. These results identify an intriguing link between genetic variations linked with disease susceptibility and their association with regions of the genome that undergo epigenetic modifications in response to nutritional challenges, implying a causal relationship. The close connection between genetic and epigenetic variation may signify their essential roles in generating individual variation [65, 66]. However, while these findings suggest that DNA methylation may be a mediator of genetic effects, it is also important to consider that both genetic and epigenetic processes could act independently on the same genes. Twin studies [8, 63, 67] can provide important insights and indicate that inter-individual differences in levels of DNA methylation arise predominantly from non-shared environment and stochastic influences, minimally from shared environmental effects, but also with a significant impact of genetic variation.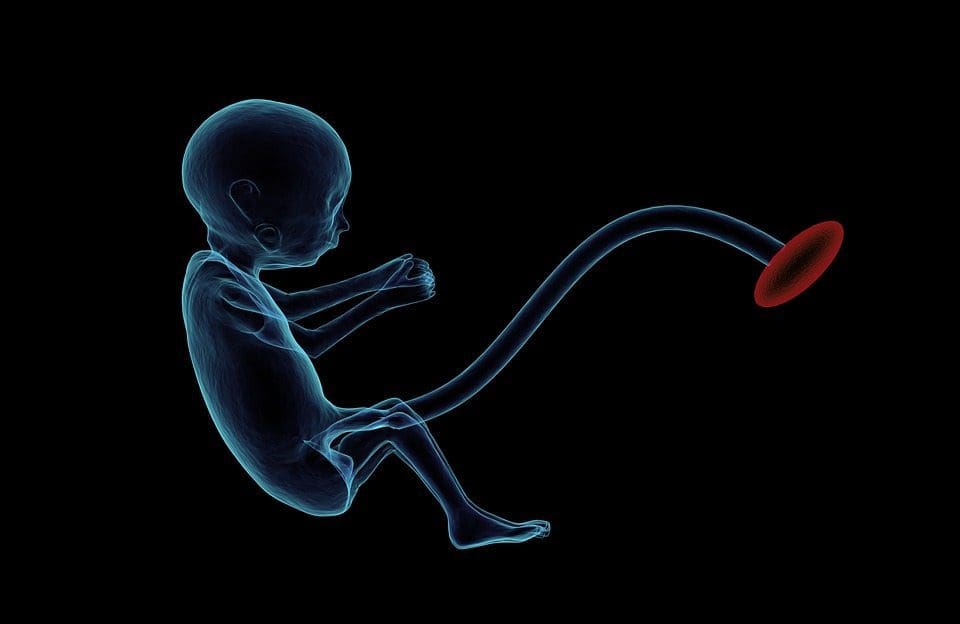 Prenatal environment: Two recently published studies made use of human populations that experienced �natural� variations in nutrient supply to study the impact of maternal nutrition before or during pregnancy on DNA methylation in the offspring [68, 69]. The first study used a Gambian mother-child cohort to show that both seasonal variations in maternal methyl donor intake during pregnancy and maternal pre-pregnancy BMI were associated with altered methylation in the infants [69]. The second study utilized adult offspring from the Dutch Hunger Winter cohort to investigate the effect of prenatal exposure to an acute period of severe maternal undernutrition on DNA methylation of genes involved in growth and metabolism in adulthood [68]. The results highlighted the importance of the timing of the exposure in its impact on the epigenome, since significant epigenetic effects were only identified in individuals exposed to famine during early gestation. Importantly, the epigenetic changes occurred in conjunction with increased BMI; however, it was not possible to establish in this study whether these changes were present earlier in life or a consequence of the higher BMI.
Prenatal environment: Two recently published studies made use of human populations that experienced �natural� variations in nutrient supply to study the impact of maternal nutrition before or during pregnancy on DNA methylation in the offspring [68, 69]. The first study used a Gambian mother-child cohort to show that both seasonal variations in maternal methyl donor intake during pregnancy and maternal pre-pregnancy BMI were associated with altered methylation in the infants [69]. The second study utilized adult offspring from the Dutch Hunger Winter cohort to investigate the effect of prenatal exposure to an acute period of severe maternal undernutrition on DNA methylation of genes involved in growth and metabolism in adulthood [68]. The results highlighted the importance of the timing of the exposure in its impact on the epigenome, since significant epigenetic effects were only identified in individuals exposed to famine during early gestation. Importantly, the epigenetic changes occurred in conjunction with increased BMI; however, it was not possible to establish in this study whether these changes were present earlier in life or a consequence of the higher BMI. Postnatal environment: The epigenome is established de novo during embryonic development, and therefore, the prenatal environment most likely has the most significant impact on the epigenome. However, it is now clear that changes do occur in the �mature� epigenome under the influence of a range of conditions, including aging, exposure to toxins, and dietary alterations. For example, changes in DNA methylation in numerous genes in skeletal muscle and PGC1A in adipose tissue have been demonstrated in response to a high-fat diet [75, 76]. Interventions to lose body fat mass have also been associated with changes in DNA methylation. Studies have reported that the DNA methylation profiles of adipose tissue [43, 64], peripheral blood mononuclear cells [77], and muscle tissue [78] in formerly obese patients become more similar to the profiles of lean subjects following weight loss. Weight loss surgery also partially reversed non-alcoholic fatty liver disease-associated methylation changes in liver [79] and in another study led to hypomethylation of multiple obesity candidate genes, with more pronounced effects in subcutaneous compared to omental (visceral) fat [64]. Accumulating evidence suggests that exercise interventions can also influence DNA methylation. Most of these studies have been conducted in lean individuals [80�82], but one exercise study in obese T2DM subjects also demonstrated changes in DNA methylation, including in genes involved in fatty acid and glucose transport [83]. Epigenetic changes also occur with aging, and recent data suggest a role of obesity in augmenting them [9, 84, 85]. Obesity accelerated the epigenetic age of liver tissue, but in contrast to the findings described above, this effect was not reversible after weight loss [84].
Postnatal environment: The epigenome is established de novo during embryonic development, and therefore, the prenatal environment most likely has the most significant impact on the epigenome. However, it is now clear that changes do occur in the �mature� epigenome under the influence of a range of conditions, including aging, exposure to toxins, and dietary alterations. For example, changes in DNA methylation in numerous genes in skeletal muscle and PGC1A in adipose tissue have been demonstrated in response to a high-fat diet [75, 76]. Interventions to lose body fat mass have also been associated with changes in DNA methylation. Studies have reported that the DNA methylation profiles of adipose tissue [43, 64], peripheral blood mononuclear cells [77], and muscle tissue [78] in formerly obese patients become more similar to the profiles of lean subjects following weight loss. Weight loss surgery also partially reversed non-alcoholic fatty liver disease-associated methylation changes in liver [79] and in another study led to hypomethylation of multiple obesity candidate genes, with more pronounced effects in subcutaneous compared to omental (visceral) fat [64]. Accumulating evidence suggests that exercise interventions can also influence DNA methylation. Most of these studies have been conducted in lean individuals [80�82], but one exercise study in obese T2DM subjects also demonstrated changes in DNA methylation, including in genes involved in fatty acid and glucose transport [83]. Epigenetic changes also occur with aging, and recent data suggest a role of obesity in augmenting them [9, 84, 85]. Obesity accelerated the epigenetic age of liver tissue, but in contrast to the findings described above, this effect was not reversible after weight loss [84].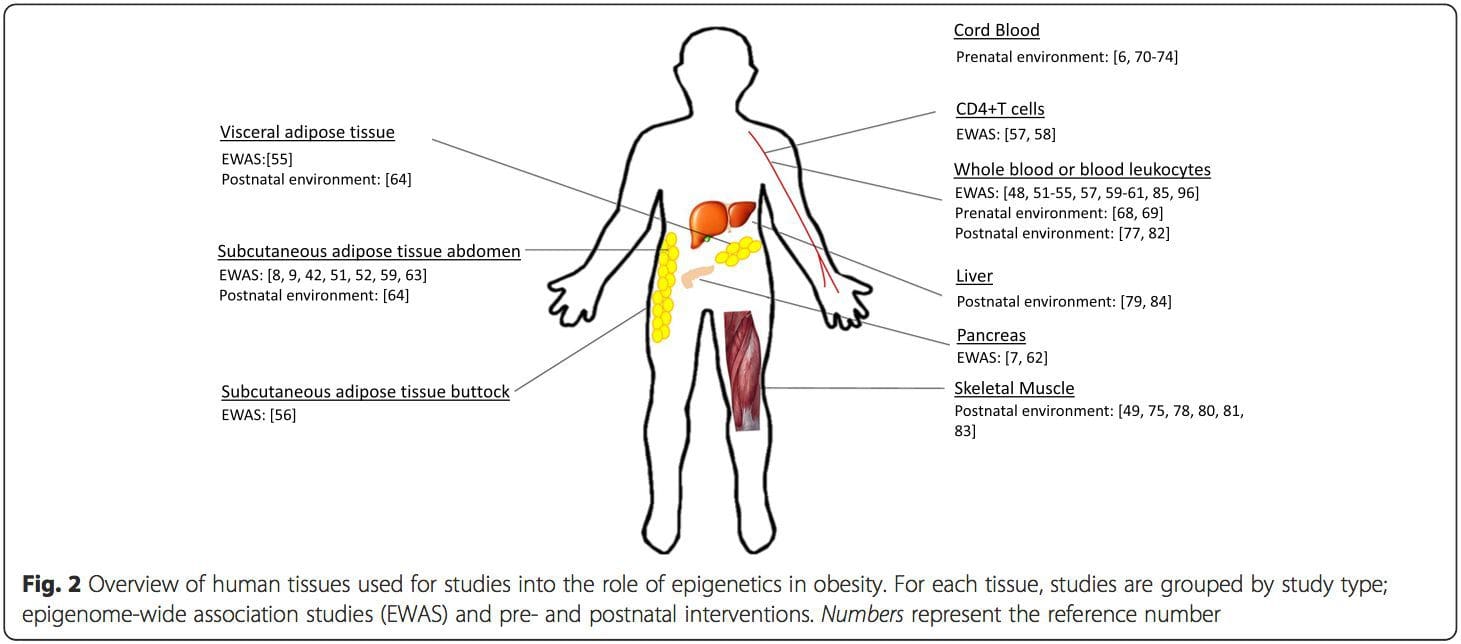 Conclusions
Conclusions

 Nutrition is increasingly recognized as a key component of optimal sporting performance, with both the science and practice of sports nutrition developing rapidly.1 Recent studies have found that a planned scientific nutritional strategy (consisting of fluid, carbohydrate, sodium, and caffeine) compared with a self-chosen nutritional strategy helped non-elite runners complete a marathon run faster2 and trained cyclists complete a time trial faster.3 Whereas training has the greatest potential to increase performance, it has been estimated that consumption of a carbohydrate�electrolyte drink or relatively low doses of caffeine may improve a 40 km cycling time trial performance by 32�42 and 55�84 seconds, respectively.4
Nutrition is increasingly recognized as a key component of optimal sporting performance, with both the science and practice of sports nutrition developing rapidly.1 Recent studies have found that a planned scientific nutritional strategy (consisting of fluid, carbohydrate, sodium, and caffeine) compared with a self-chosen nutritional strategy helped non-elite runners complete a marathon run faster2 and trained cyclists complete a time trial faster.3 Whereas training has the greatest potential to increase performance, it has been estimated that consumption of a carbohydrate�electrolyte drink or relatively low doses of caffeine may improve a 40 km cycling time trial performance by 32�42 and 55�84 seconds, respectively.4
 Carbohydrate ingestion has been shown to improve performance in events lasting approximately 1 hour.6 A growing body of evidence also demonstrates beneficial effects of a carbohydrate mouth rinse on performance.22 It is thought that receptors in the oral cavity signal to the central nervous system to positively modify motor output.23
Carbohydrate ingestion has been shown to improve performance in events lasting approximately 1 hour.6 A growing body of evidence also demonstrates beneficial effects of a carbohydrate mouth rinse on performance.22 It is thought that receptors in the oral cavity signal to the central nervous system to positively modify motor output.23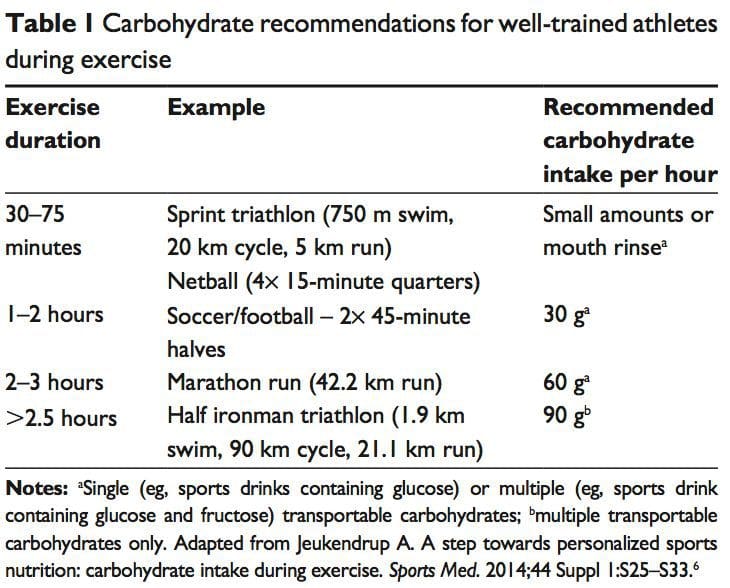
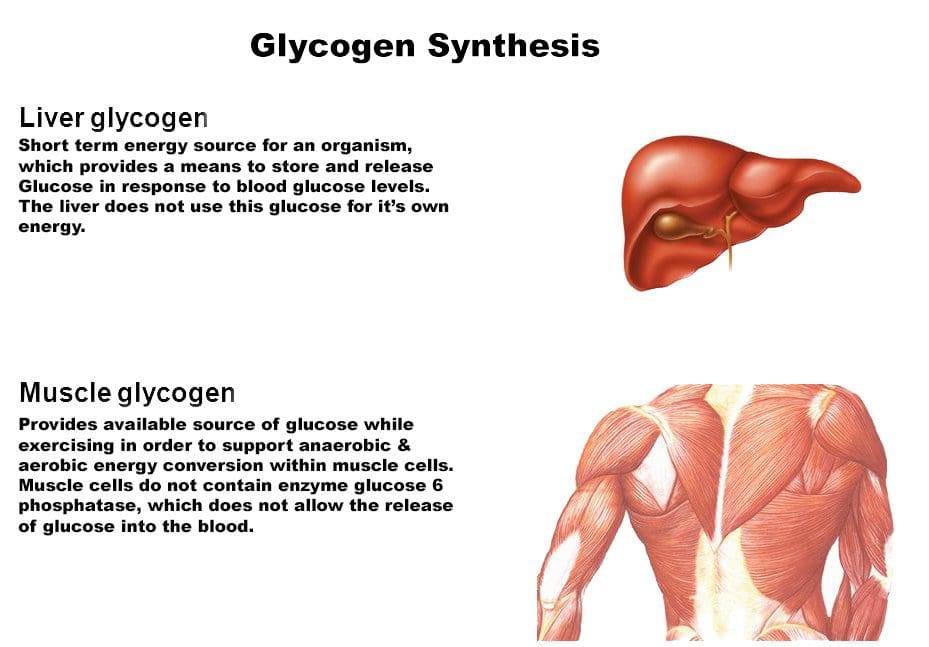
 The �train-low, compete-high� concept is training with low carbohydrate availability to promote adaptations such as�enhanced activation of cell-signaling pathways, increased mitochondrial enzyme content and activity, enhanced lipid oxidation rates, and hence improved exercise capacity.26 However, there is no clear evidence that performance is improved with this approach.27 For example, when highly trained cyclists were separated into once-daily (train-high) or twice-daily (train-low) training sessions, increases in resting muscle glycogen content were seen in the low-carbohydrate- availability group, along with other selected training adaptations.28 However, performance in a 1-hour time trial after 3 weeks of training was no different between groups. Other research has produced similar results.29 Different strategies have been suggested (eg, training after an overnight fast, training twice per day, restricting carbohydrate during recovery),26 but further research is needed to establish optimal dietary periodization plans.27
The �train-low, compete-high� concept is training with low carbohydrate availability to promote adaptations such as�enhanced activation of cell-signaling pathways, increased mitochondrial enzyme content and activity, enhanced lipid oxidation rates, and hence improved exercise capacity.26 However, there is no clear evidence that performance is improved with this approach.27 For example, when highly trained cyclists were separated into once-daily (train-high) or twice-daily (train-low) training sessions, increases in resting muscle glycogen content were seen in the low-carbohydrate- availability group, along with other selected training adaptations.28 However, performance in a 1-hour time trial after 3 weeks of training was no different between groups. Other research has produced similar results.29 Different strategies have been suggested (eg, training after an overnight fast, training twice per day, restricting carbohydrate during recovery),26 but further research is needed to establish optimal dietary periodization plans.27 There has been a recent resurgence of interest in fat as a fuel, particularly for ultra endurance exercise. A high-carbohydrate strategy inhibits fat utilization during exercise,30 which may not be beneficial due to the abundance of energy stored in the body as fat. Creating an environment that optimizes fat oxidation potentially occurs when dietary carbohydrate is reduced to a level that promotes ketosis.31 However, this strategy may impair performance of high-intensity activity, by contributing to a reduction in pyruvate dehydrogenase activity and glycogenolysis. 32 The lack of performance benefits seen in studies investigating �high-fat� diets may be attributed to inadequate carbohydrate restriction and time for adaptation.31 Research into the performance effects of high fat diets continues.
There has been a recent resurgence of interest in fat as a fuel, particularly for ultra endurance exercise. A high-carbohydrate strategy inhibits fat utilization during exercise,30 which may not be beneficial due to the abundance of energy stored in the body as fat. Creating an environment that optimizes fat oxidation potentially occurs when dietary carbohydrate is reduced to a level that promotes ketosis.31 However, this strategy may impair performance of high-intensity activity, by contributing to a reduction in pyruvate dehydrogenase activity and glycogenolysis. 32 The lack of performance benefits seen in studies investigating �high-fat� diets may be attributed to inadequate carbohydrate restriction and time for adaptation.31 Research into the performance effects of high fat diets continues. While protein consumption prior to and during endurance and resistance exercise has been shown to enhance rates of muscle protein synthesis (MPS), a recent review found protein ingestion alongside carbohydrate during exercise does not improve time�trial performance when compared with the ingestion of adequate amounts of carbohydrate alone.33
While protein consumption prior to and during endurance and resistance exercise has been shown to enhance rates of muscle protein synthesis (MPS), a recent review found protein ingestion alongside carbohydrate during exercise does not improve time�trial performance when compared with the ingestion of adequate amounts of carbohydrate alone.33 The purpose of fluid consumption during exercise is primarily to maintain hydration and thermoregulation, thereby benefiting performance. Evidence is emerging on increased risk of oxidative stress with dehydration.34 Fluid consumption prior to exercise is recommended to ensure that the athlete is well-hydrated prior to commencing exercise.35 In addition,�carefully planned hyperhydration ( fluid overloading) prior to an event may reset fluid balance and increase fluid retention, and consequently improve heat tolerance.36 However, fluid overloading may increase the risk of hyponatremia 37 and impact negatively on performance due to feelings of fullness and the need to urinate.
The purpose of fluid consumption during exercise is primarily to maintain hydration and thermoregulation, thereby benefiting performance. Evidence is emerging on increased risk of oxidative stress with dehydration.34 Fluid consumption prior to exercise is recommended to ensure that the athlete is well-hydrated prior to commencing exercise.35 In addition,�carefully planned hyperhydration ( fluid overloading) prior to an event may reset fluid balance and increase fluid retention, and consequently improve heat tolerance.36 However, fluid overloading may increase the risk of hyponatremia 37 and impact negatively on performance due to feelings of fullness and the need to urinate. Performance supplements shown to enhance performance include caffeine, beetroot juice, beta-alanine (BA), creatine, and bicarbonate.40 Comprehensive reviews on other supplements including caffeine, creatine, and bicarbonate can be found elsewhere.41 In recent years, research has focused on the role of nitrate, BA, and vitamin D and performance. Nitrate is most commonly provided as sodium nitrate or beetroot juice.42 Dietary nitrates are reduced (in mouth and stomach) to nitrites, and then to nitric oxide. During exercise, nitric oxide potentially influences skeletal muscle function through regulation of blood ow and glucose homeostasis, as well as mitochondrial respiration.43 During endurance exercise, nitrate supplementation has been shown to increase exercise efficiency (4%�5% reduction in VO at a steady attenuate oxidative stress.42 Similarly, a 4.2% improvement in performance was shown in a test designed to simulate a football game.44
Performance supplements shown to enhance performance include caffeine, beetroot juice, beta-alanine (BA), creatine, and bicarbonate.40 Comprehensive reviews on other supplements including caffeine, creatine, and bicarbonate can be found elsewhere.41 In recent years, research has focused on the role of nitrate, BA, and vitamin D and performance. Nitrate is most commonly provided as sodium nitrate or beetroot juice.42 Dietary nitrates are reduced (in mouth and stomach) to nitrites, and then to nitric oxide. During exercise, nitric oxide potentially influences skeletal muscle function through regulation of blood ow and glucose homeostasis, as well as mitochondrial respiration.43 During endurance exercise, nitrate supplementation has been shown to increase exercise efficiency (4%�5% reduction in VO at a steady attenuate oxidative stress.42 Similarly, a 4.2% improvement in performance was shown in a test designed to simulate a football game.44
 Consuming carbohydrates immediately
Consuming carbohydrates immediately  An acute bout of intense endurance or resistance exercise can induce a transient increase in protein turnover, and, until feeding, protein balance remains negative. Protein consumption after exercise enhances MPS and net protein balance,58 predominantly by increasing mitochondrial protein fraction with endurance training, and myofibrillar protein fraction with resistance training.59
An acute bout of intense endurance or resistance exercise can induce a transient increase in protein turnover, and, until feeding, protein balance remains negative. Protein consumption after exercise enhances MPS and net protein balance,58 predominantly by increasing mitochondrial protein fraction with endurance training, and myofibrillar protein fraction with resistance training.59 Fluid and electrolyte replacement after exercise can be achieved through resuming normal hydration practices. However, when euhydration is needed within 24 hours or substantial body weight has been lost (.5% of BM), a more structured response may be warranted to replace fluids and electrolytes.77
Fluid and electrolyte replacement after exercise can be achieved through resuming normal hydration practices. However, when euhydration is needed within 24 hours or substantial body weight has been lost (.5% of BM), a more structured response may be warranted to replace fluids and electrolytes.77 The availability of nutrition information for athletes varies. Younger or recreational athletes are more likely to receive generalized nutritional information of poorer quality from individuals such as coaches.78 Elite athletes are more likely to have access to specialized sports-nutrition input from qualified professionals. A range of sports science and medicine support systems are in place in different countries to assist elite athletes,1 and nutrition is a key component of these services. Some countries have nutrition programs embedded within sports institutes (eg, Australia) or alternatively have National Olympic Committees that support nutrition programs (eg, United States of America).1 However, not all athletes at the elite level have access to sports-nutrition services. This may be due to financial constraints of the sport, geographical issues, and a lack of recognition of the value of a sports-nutrition service.78
The availability of nutrition information for athletes varies. Younger or recreational athletes are more likely to receive generalized nutritional information of poorer quality from individuals such as coaches.78 Elite athletes are more likely to have access to specialized sports-nutrition input from qualified professionals. A range of sports science and medicine support systems are in place in different countries to assist elite athletes,1 and nutrition is a key component of these services. Some countries have nutrition programs embedded within sports institutes (eg, Australia) or alternatively have National Olympic Committees that support nutrition programs (eg, United States of America).1 However, not all athletes at the elite level have access to sports-nutrition services. This may be due to financial constraints of the sport, geographical issues, and a lack of recognition of the value of a sports-nutrition service.78 Supplement use is widespread in athletes.86,87 For example, 87.5% of elite athletes in Australia used dietary supplements88 and 87% of Canadian high-performance athletes took dietary supplements within the past 6 months85 (Table 2). It is difficult to compare studies due to differences in the criteria used to define dietary supplements, variations in assessing supplement intake, and disparities in the populations studied.85
Supplement use is widespread in athletes.86,87 For example, 87.5% of elite athletes in Australia used dietary supplements88 and 87% of Canadian high-performance athletes took dietary supplements within the past 6 months85 (Table 2). It is difficult to compare studies due to differences in the criteria used to define dietary supplements, variations in assessing supplement intake, and disparities in the populations studied.85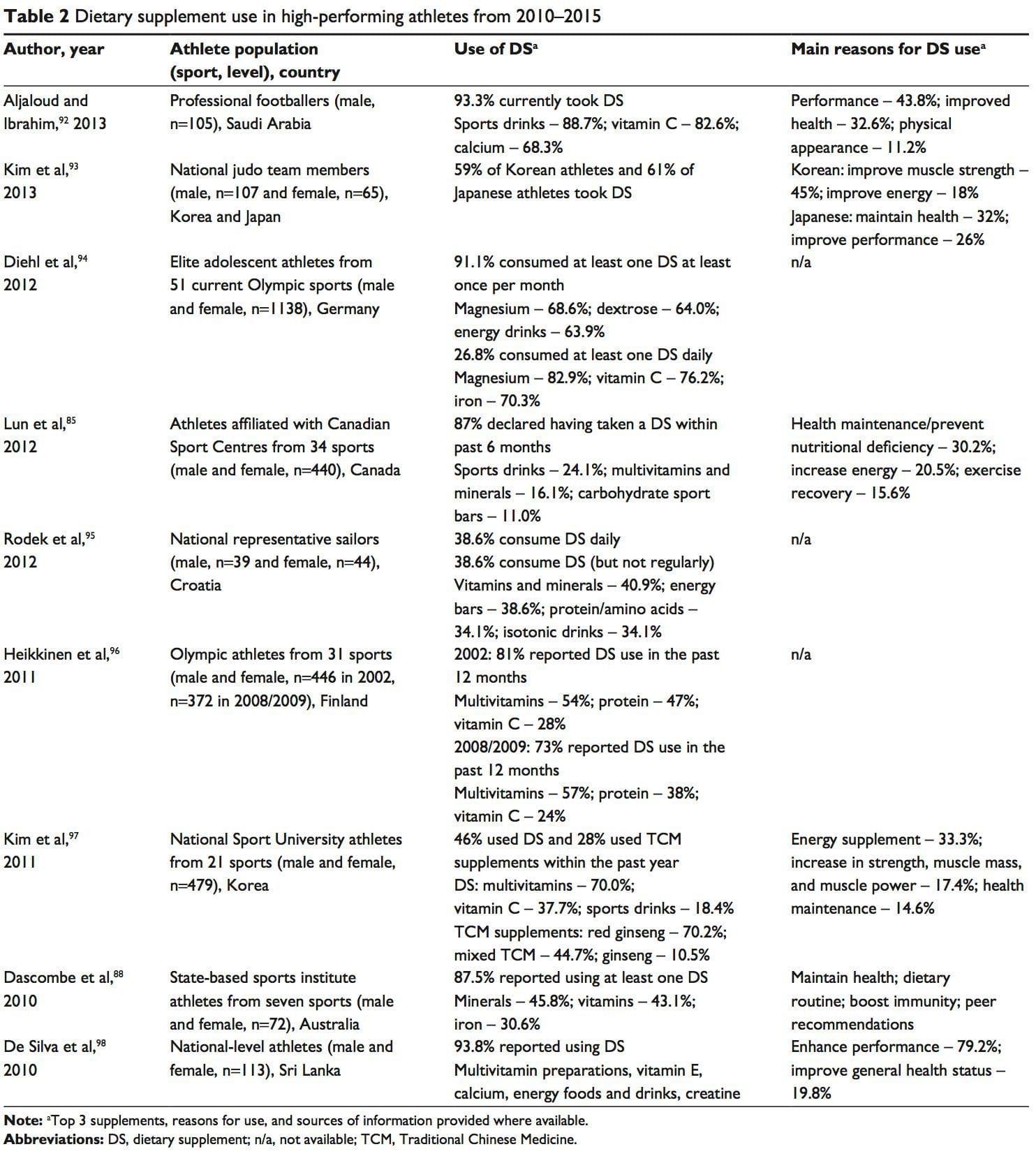 A positive drug test in an athlete can occur with even a minute quantity of a banned substance.41,87 WADA maintains a �strict liability� policy, whereby every athlete is responsible for any substance found in their body regardless of how it got there.41,86,87,89 The World Anti-Doping Code (January 1, 2015) does recognize the issue of contaminated supplements.91 Whereas the code upholds the principle of strict liability, athletes may receive a lesser ban if they can��show �no significant fault� to demonstrate they did not intend to cheat. The updated code imposes longer bans on those who cheat intentionally, includes athlete support personnel (eg, coaches, medical staff), and has an increased focus on anti-doping education.91,99
A positive drug test in an athlete can occur with even a minute quantity of a banned substance.41,87 WADA maintains a �strict liability� policy, whereby every athlete is responsible for any substance found in their body regardless of how it got there.41,86,87,89 The World Anti-Doping Code (January 1, 2015) does recognize the issue of contaminated supplements.91 Whereas the code upholds the principle of strict liability, athletes may receive a lesser ban if they can��show �no significant fault� to demonstrate they did not intend to cheat. The updated code imposes longer bans on those who cheat intentionally, includes athlete support personnel (eg, coaches, medical staff), and has an increased focus on anti-doping education.91,99