
Functional Medicine: Consolidated Glossary
Functional Medicine: Glossary
 Allostasis: The process of achieving stability, or homeostasis, through physiological or behavioral change. This can be carried out by means of alteration in HPATG axis hormones, the autonomic nervous system, cytokines, or a number of other systems, and is generally adaptive in the short term. It is essential in order to maintain internal viability amid changing conditions.
Allostasis: The process of achieving stability, or homeostasis, through physiological or behavioral change. This can be carried out by means of alteration in HPATG axis hormones, the autonomic nervous system, cytokines, or a number of other systems, and is generally adaptive in the short term. It is essential in order to maintain internal viability amid changing conditions.
Antecedents: Factors that predispose to acute or chronic illness. For a person who is ill, antecedents form the illness diathesis. From the perspective of prevention, they are risk factors. Examples of genetic antecedents include the breast cancer risk genes BRCA1 and BRCA2.
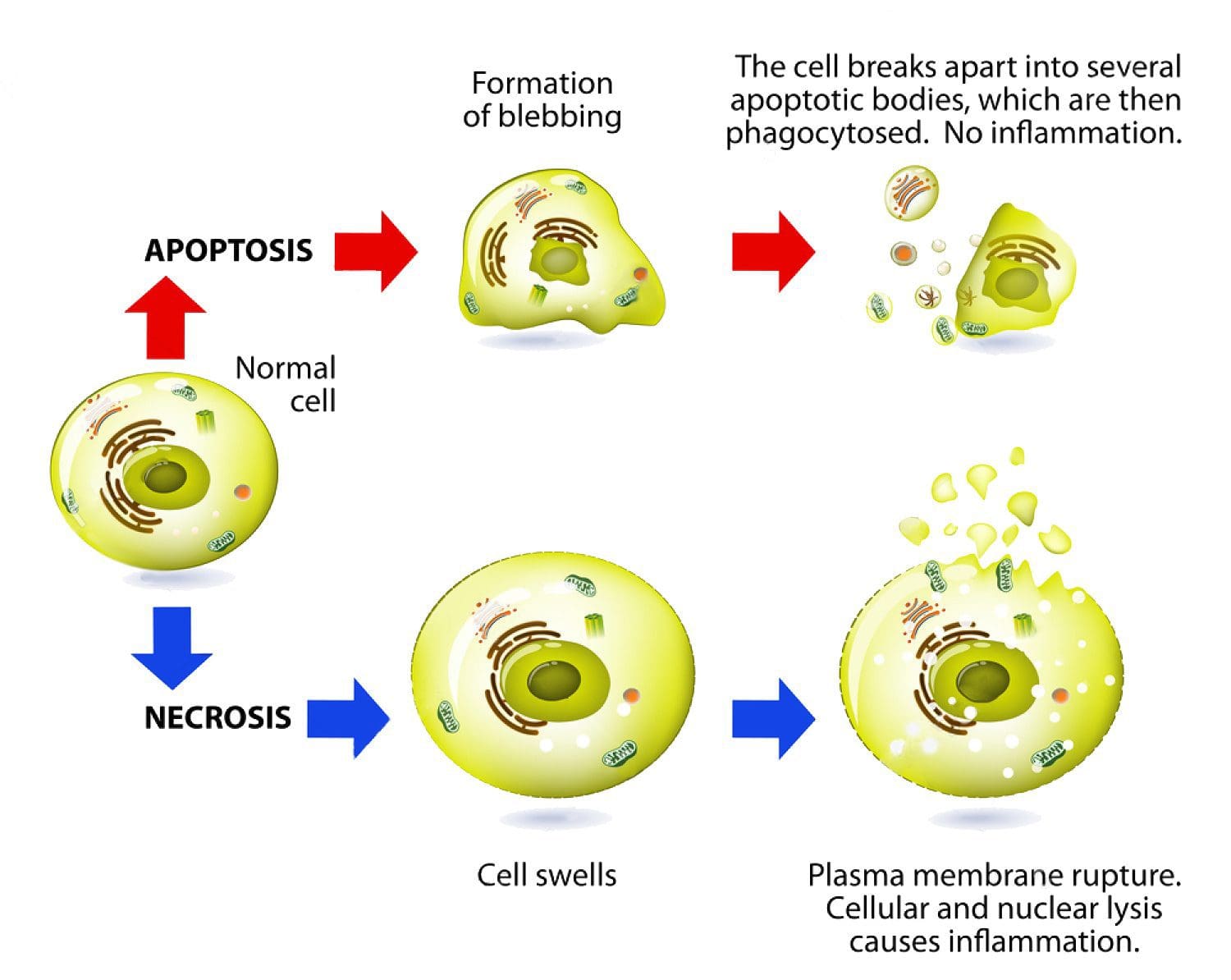
Apoptosis: Programmed cell death. As a normal part of growth and development, cells that are superfluous or that become damaged activate a cascade of intracellular processes leading to their own demise. In cancer cells, DNA damage may inactivate the apoptosis cascade, allowing mutated cells to survive and proliferate.
Biochemical individuality: Each individual has a unique physiological and biochemical composition, based upon the interactions of his or her individual genetic make-up with lifestyle and environment�i.e., the continuous exposure to inputs (diet, experiences, nutrients, beliefs, activity, toxins, medications, etc.) that influence our genes. It is this combination of factors that accounts for the endless variety of phenotypic responses seen every day by clinicians. The unique makeup of each individual requires personalized levels of nutrition and a lifestyle adapted to that individual�s needs in order to achieve optimal health. The consequences of not meeting the specific needs of the individual are expressed, over time, as degenerative disease.�
Bioidentical Hormone Therapy: Giving exogenous hormones that are identical in structure to the endogenous hormones.�
Biomarker: A substance used as an indicator of a biological state. Such characteristics are objectively measured and evaluated as indicators of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. Cancer biomarkers include prostate specific antigen (PSA) and carcinoembryonic antigen (CEA).
Biotransformation: The chemical modification(s) of a compound made by an organism. Compounds modified in the body include, but are not limited to, nutrients, amino acids, toxins, heavy metals, and drugs. Biotransformation also renders nonpolar compounds polar so that they are excreted, not reabsorbed in renal tubules.
Cancer: A group of diseases characterized by uncontrolled growth and spread of abnormal cells, which, if not controlled, can result in death. Cancer is caused by both external factors (tobacco, infectious organisms, chemicals, and radiation) and internal factors (inherited mutations, hormones, immune conditions, and mutations that occur from metabolism), two or more of which may act together or in sequence to initiate or promote carcinogenesis. Ten or more years often pass between exposure to external factors and detectable cancer.
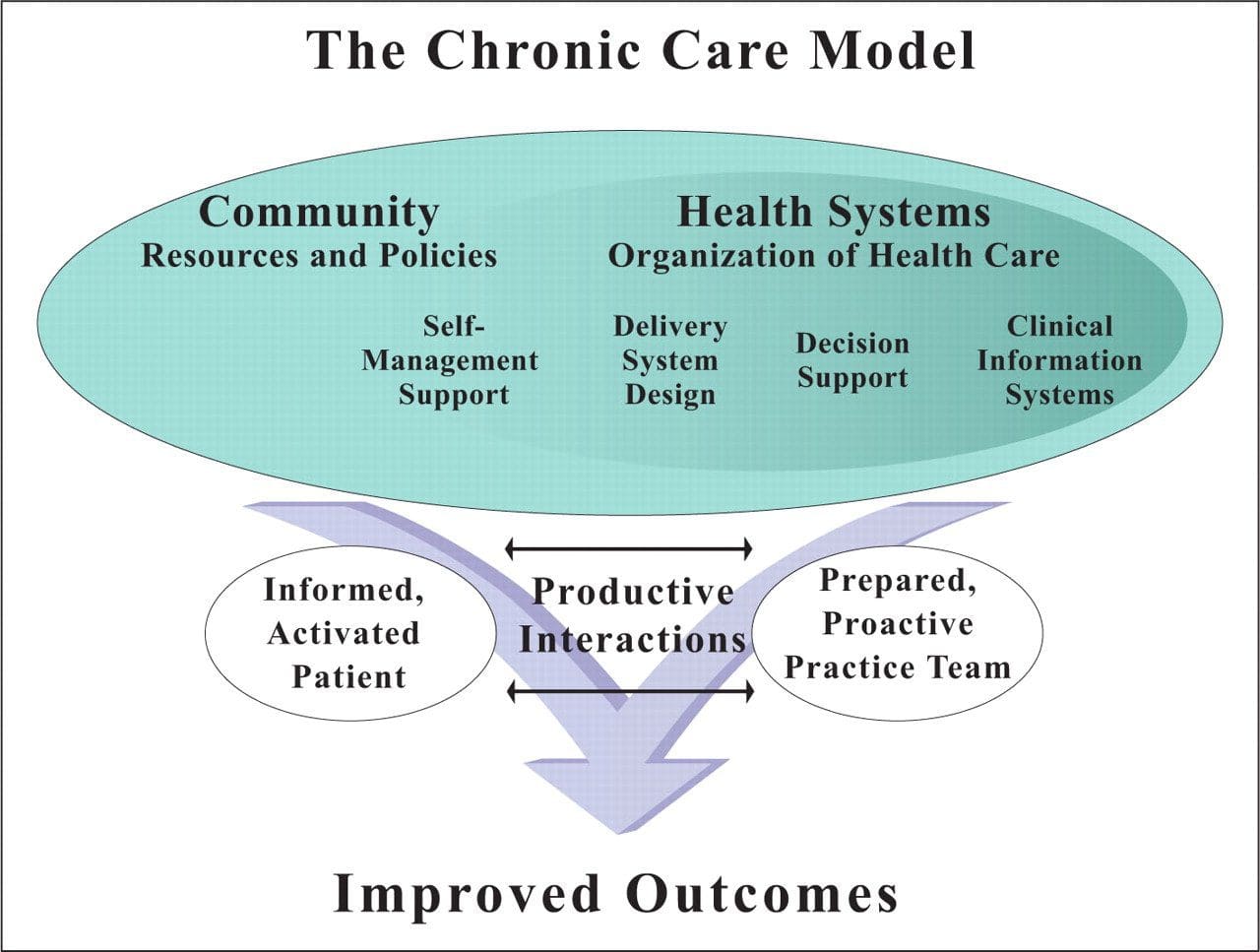 Chronic Care Model: Developed by Wagner and colleagues, the primary focus of this model is to include the essential elements of a healthcare system that encourage high-quality chronic disease care. Such elements include the community, the health system, self-management support, delivery system design, decision support and clinical information systems. It is a response to powerful evidence that patients with chronic conditions often do not obtain the care they need, and that the healthcare system is not currently structured to facilitate such care.�
Chronic Care Model: Developed by Wagner and colleagues, the primary focus of this model is to include the essential elements of a healthcare system that encourage high-quality chronic disease care. Such elements include the community, the health system, self-management support, delivery system design, decision support and clinical information systems. It is a response to powerful evidence that patients with chronic conditions often do not obtain the care they need, and that the healthcare system is not currently structured to facilitate such care.�
 Complementary and Alternative Medicine (CAM): A group of diverse medical and healthcare systems, practices, and products that are not presently considered to be part of conventional, mainstream medicine. The list of what is considered to be CAM changes frequently, as therapies demonstrated to be safe and effective are adopted by conventional practitioners, and as new approaches to health care emerge. Complementary medicine is used with conventional medicine, not as a substitute for it. Alternative medicine is used in place of conventional medicine. Functional medicine is neither complementary nor alternative medicine; it is an approach to medicine that focuses on identifying and ameliorating the underlying causes of disease; it can be used by all practitioners with a Western medical science background and is compatible with both conventional and CAM methods.�
Complementary and Alternative Medicine (CAM): A group of diverse medical and healthcare systems, practices, and products that are not presently considered to be part of conventional, mainstream medicine. The list of what is considered to be CAM changes frequently, as therapies demonstrated to be safe and effective are adopted by conventional practitioners, and as new approaches to health care emerge. Complementary medicine is used with conventional medicine, not as a substitute for it. Alternative medicine is used in place of conventional medicine. Functional medicine is neither complementary nor alternative medicine; it is an approach to medicine that focuses on identifying and ameliorating the underlying causes of disease; it can be used by all practitioners with a Western medical science background and is compatible with both conventional and CAM methods.�
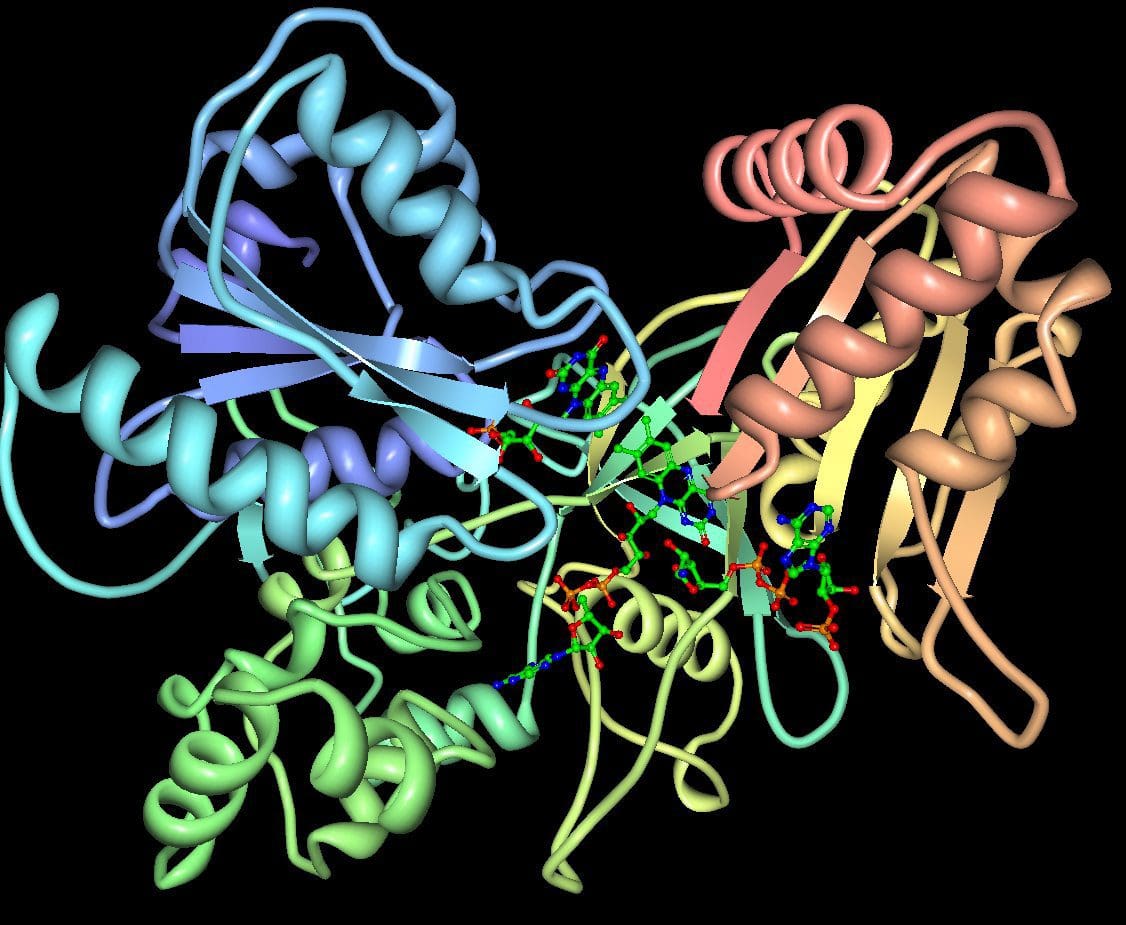 Cytochromes P450 (CYP 450): A large and diverse group of enzymes, most of which function to catalyze the oxidation of organic substances. They are located either in the inner membrane of mitochondria or in the endoplasmic reticulum of cells ans play a critical role in the detoxification of endogenous and exogenous toxins. The substrates of CYP enzymes include metabolic intermediates such as lipids, steroidal hormones, and xenobiotic substances such as drugs.
Cytochromes P450 (CYP 450): A large and diverse group of enzymes, most of which function to catalyze the oxidation of organic substances. They are located either in the inner membrane of mitochondria or in the endoplasmic reticulum of cells ans play a critical role in the detoxification of endogenous and exogenous toxins. The substrates of CYP enzymes include metabolic intermediates such as lipids, steroidal hormones, and xenobiotic substances such as drugs.
DIGIN: A heuristic mnemonic for assessment of gastrointestinal dysfunction. Thorough assessment of the GI tract should include investigation of the following:
- Digestion/Absorption � Problems with the digestive process including ingestion, chemical digestion, mechanical digestion, absorption, and/or assimilation
- Intestinal Permeability � Permeability of the intestinal barrier: is the epithelium allowing in larger particles in a paracellular manner, making the gut barrier �leaky�?
- Gut Microbiota/Dysbiosis � Changes in composition of the gut flora including balance and interaction of commensal species (See: Dysbiosis)
- Inflammation/Immune � Inflammation and immune activity in the GI tract
- Nervous System � Enteric nervous system function, which controls motility, blood flow, uptake of nutrients, secretion, and immunological and inflammatory processes in the gut.
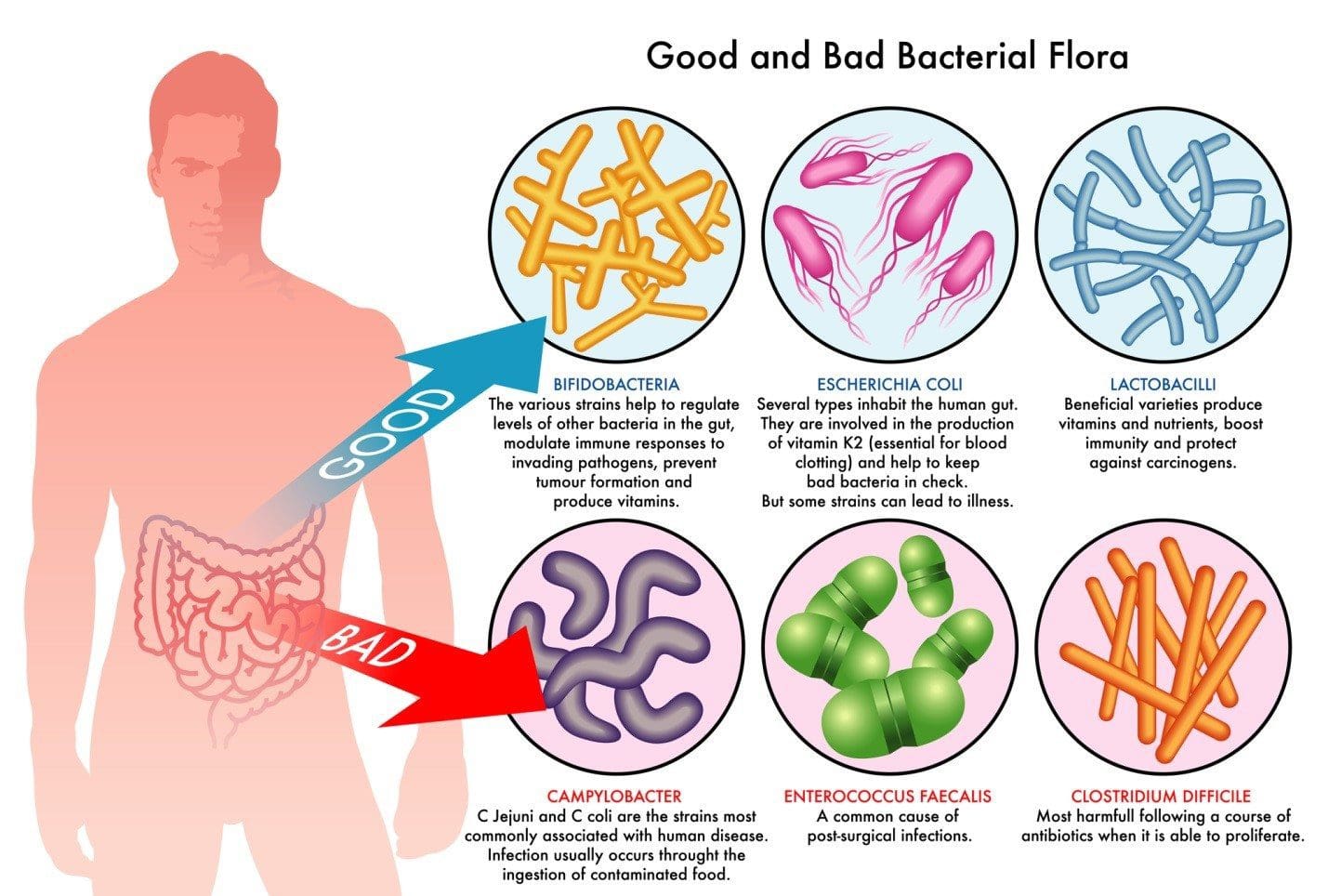 Dysbiosis: A condition that occurs when the normal symbiosis between gut flora and the host is disturbed and organisms of low intrinsic virulence, which normally coexist peacefully with the host, may promote illness. It is distinct from gastrointestinal infection, in which a highly virulent organism gains access to the gastrointestinal tract and infects the host.�
Dysbiosis: A condition that occurs when the normal symbiosis between gut flora and the host is disturbed and organisms of low intrinsic virulence, which normally coexist peacefully with the host, may promote illness. It is distinct from gastrointestinal infection, in which a highly virulent organism gains access to the gastrointestinal tract and infects the host.�
Functional Medicine: A systems-based, science-driven approach to individualized medicine that addresses the underlying causes of disease, using a systems-oriented approach and engaging both patient and practitioner in a therapeutic partnership. It reflects a personalized lifestyle medicine approach and utilizes the Functional Medicine Matrix to organize the patient�s story and determine appropriate interventions for the prevention and treatment of chronic diseases.
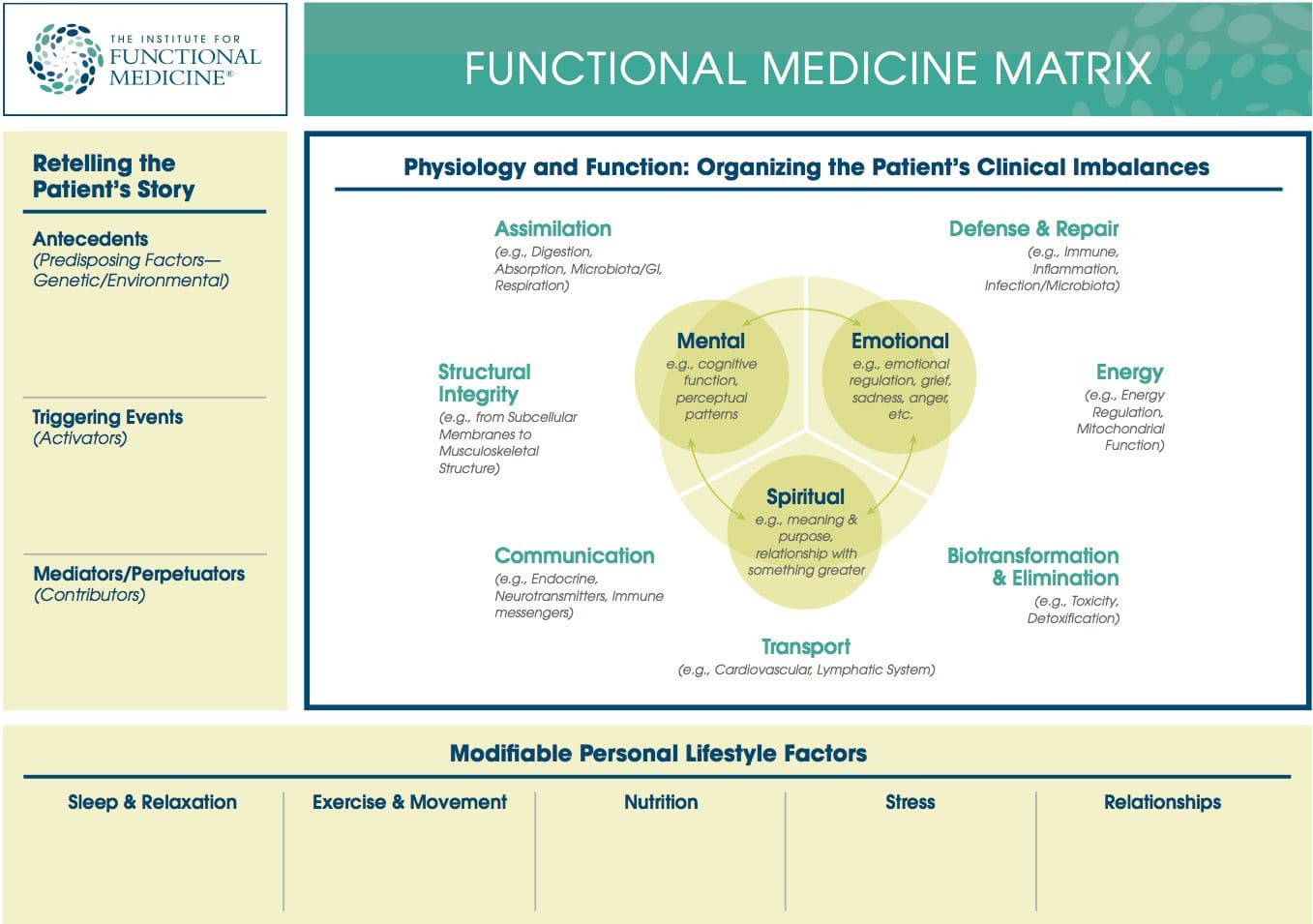 Functional Medicine Matrix: The graphic representation of the functional medicine approach, displaying the seven organizing physiological systems, the patient�s known antecedents, triggers, and mediators, and the personalized lifestyle factors that promote health. Practitioners can use the matrix to help organize their thoughts and observations about the patient�s health and decide how to focus therapeutic and preventive strategies.
Functional Medicine Matrix: The graphic representation of the functional medicine approach, displaying the seven organizing physiological systems, the patient�s known antecedents, triggers, and mediators, and the personalized lifestyle factors that promote health. Practitioners can use the matrix to help organize their thoughts and observations about the patient�s health and decide how to focus therapeutic and preventive strategies.
Cytokines: Immunoregulatory proteins (such as interleukin, tumor necrosis factor, and interferon). They may act locally or systemically and tend to have both immunomodulatory and other effects on cellular processes in the body. Cytokines have been used in the treatment of certain cancers.�
Genomics: The study of the whole genome of organisms, including interactions between loci and alleles within the genome. Research on single genes does not fall into the definition of genomics unless the aim of this functional information analysis is to elucidate the gene�s effect on the entire genome network. Genomics may also be defined as the study of all the genes of a cell, or tissue, at the DNA (genotype), mRNA (transcriptome), or protein (proteome) levels.�
GO-TO-IT: A heuristic mnemonic for the processes involved in the clinical practice of functional medicine:
- Gather oneself and be mindful in preparing to see each patient; gather information through intake forms, questionnaires, the initial consultation, physical exam, and objective data. A detailed functional medicine history that is appropriate to age, gender, and nature of presenting problems is taken.
- Organize the subjective and objective details from the patient�s story within the functional medicine paradigm. Position the patient�s presenting signs and symptoms, along with the details of the case history, on the timeline and Functional Medicine Matrix.
- Tell the story back to the patient in your own words to ensure accuracy and understanding. The re-telling of the patient�s story is a dialogue about the case highlights�including the antecedents, triggers, and mediators identified in the history and correlating them to the timeline and matrix. The patient is asked to correct and amplify the story, engendering a context of true partnership.
- Order and then prioritize the patient�s information:
- Acknowledge patient�s goals
- Address modifiable lifestyle factors
- Sidney Baker�s too much/not enough model: what are the insufficiencies/excesses?
- Identify clinical imbalances or disruptions in the organizing physiological systems of the matrix
- Initiate further functional assessment and intervention based upon the above work:
- Perform further assessment
- Referral to adjunctive care:
- Nutritional professionals
- Lifestyle educators
- Other healthcare providers
- Specialists
- Initiate therapy
- Track assessments, note the effectiveness of the therapeutic approach, and identify clinical outcomes at each visit�in partnership with the patient.
Heuristic: A strategy used for problem solving, learning, and discovery that is experience-based, not algorithmic. When an exhaustive search is impractical, heuristic methods may be used to speed up the process of finding a satisfactory solution. A heuristic is sometimes referred to as a rule of thumb.
Homeostasis and Homeodynamics: The former term describes the tendency of living things to maintain physiological parameters within a narrow range usually considered normal in order to maintain optimal function. Under this definition, disease can be defined as a departure from the homeostatic state. The latter term describes the tendency of homeostatic set points to change throughout an organism�s lifespan, and thus describes how departures from a homeostatic norm can be adaptive (e.g., fever) or pathological, depending on the context.
Integrative Medicine: Medicine that combines treatments from conventional medicine and those from Complementary and Alternative Medicine (CAM) for which there is some high-quality evidence of safety and effectiveness. In a broader sense, it is healing-oriented medicine that takes into account the whole person (body, mind, and spirit), including all aspects of lifestyle, and makes use of all appropriate therapies, both conventional and alternative. The field is more than 10 years old and it is the only one of the emerging models to explicitly encompass the integration of therapeutics that, until recently, were the sole purview of complementary and alternative medicine. Note: functional medicine is different from integrative medicine because functional medicine emphasizes the evaluation of underlying causes of health and dysfunction and organizes assessment and treatment using the Functional Medicine Matrix, the timeline, and GOTOIT.
 Lifestyle Medicine: The use of lifestyle interventions such as nutrition, physical activity, stress reduction, and rest to lower the risk for the approximately 70% of modern health problems that are lifestyle-related chronic diseases (such as obesity and type 2 diabetes), or for the treatment and management of disease if such conditions are already present. It is an essential component of the treatment of most chronic diseases and has been incorporated in many national disease management guidelines.
Lifestyle Medicine: The use of lifestyle interventions such as nutrition, physical activity, stress reduction, and rest to lower the risk for the approximately 70% of modern health problems that are lifestyle-related chronic diseases (such as obesity and type 2 diabetes), or for the treatment and management of disease if such conditions are already present. It is an essential component of the treatment of most chronic diseases and has been incorporated in many national disease management guidelines.
Long Latency Disease: Disease that becomes manifest at a time remote from the initial exposure to disease triggers, or that requires continued exposure to triggers and mediators over an extended period of time to manifest frank pathology. Examples include heart disease, cancer, and osteoporosis.�
Mediators: Intermediaries that contribute to the continued manifestations of disease. Mediators do not cause disease; instead, they underlie the host response to triggers. Examples include biochemical factors (e.g., cytokines and leukotrienes) as well as psychosocial ones (e.g., reinforcement for staying ill).�
Metabolomics (or metabonomics): �The study of metabolic responses to drugs, environmental changes and diseases. Metabonomics is an extension of genomics (concerned with DNA) and proteomics (concerned with proteins). Following on the heels of genomics and proteomics, metabonomics may lead to more efficient drug discovery and individualized patient treatment with drugs, among other things.� (From MedicineNet.com)�
Nutrigenomics (or nutritional genomics): The study of how different foods may interact with specific genes to increase the risk of common chronic diseases such as type 2 diabetes, obesity, heart disease, stroke, and certain cancers. It can also be described as the study of the influence of genetic variation on nutrition by correlating gene expression or single-nucleotide polymorphisms with a nutrient’s absorption, metabolism, elimination, or biological effects. Nutrigenomics also seeks to provide a molecular understanding of how common chemicals in the diet affect health by altering the expression of genes and the structure of an individual’s genome. The ultimate aim of nutrigenomics is to develop rational means to optimize nutrition for the patient�s genotype.�
Organ Reserve: The difference between the maximal function of a vital organ and the level of function required to maintain an individual�s daily life. In other words, it is the �reserve power� of a particular organ, above and beyond what is required in a healthy individual. It can also be thought of as the degrees of freedom available in the body organs to perform their functions and maintain health. Decline in the organ reserve occurs under stress, during sickness, and as we age.�
Organ System Diagnosis: In the allopathic medical model, it is common to give a collection of symptoms a name based on dysfunction in an organ system, then to cite the named disease as the cause of the symptoms the patient is experiencing. This bit of circular logic avoids any discussion of the systemic or underlying causes of dysfunction and also treats all people with �disease X� the same, despite the fact that two people with the same collection of symptoms may have completely different underlying physiological causes for the symptoms they display.�
Organizing Physiological Systems: To assist clinicians in understanding and applying the complexity of functional medicine, IFM has organized and adapted a set of seven interrelated biological systems that underlie all physiology. Imbalances in these systems or core clinical imbalances are the underlying cause of disease and dysfunction.
- Assimilation (e.g., Digestion, Absorption, Microbiota/GI, Respiration)
- Defense and Repair (e.g., Immune, Inflammation, Infection/Microbiota)
- Energy (e.g., Energy Regulation, Mitochondrial Function)
- Biotransformation and Elimination (e.g., Toxicity, Detoxification)
- Transport (e.g., Circulation, Lymphatic Flow)
- Communication (e.g., Endocrine, Neurotransmitters, Immune messengers)
- Structural Integrity (e.g., from Subcellular Membranes to Musculoskeletal Structure)�
Using this construct, it becomes much clearer that one disease/condition may have multiple causes (i.e., multiple clinical imbalances), just as one fundamental imbalance may be at the root of many seemingly disparate conditions.�
Oxidation-Reduction (also called Redox): Paired chemical reactions that occur in balance with each other within the body of a healthy individual. These reactions involve the transfer of electrons (or the distribution of electron sharing) and thus require both a donor and acceptor. When this physiological parameter is out of balance, a net accumulation of donors or acceptors can lead to deleterious cellular oxidation phenomena (lipid peroxidation, free radical formation).�
Oxidative Stress: Oxidative stress occurs when there is an imbalance between the production of damaging reactive oxygen species and an individual�s antioxidant capacity to detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of tissues can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Oxidative stress is implicated in the etiology of several chronic diseases including atherosclerosis, Parkinson’s disease, Alzheimer’s disease, and chronic fatigue syndrome.�
Personalized Lifestyle Factors: The modifiable lifestyle factors that appear along the bottom of the Functional Medicine Matrix. Clinicians and their patients can partner to develop an individualized plan for addressing these issues. Health-promoting lifestyle factors include:
- Sleep and Relaxation � Getting adequate sleep and meaningful relaxation time in one�s life
- Exercise and Movement � Participating in physical activity that is appropriate for age and health
- Nutrition and Hydration � Eating a diet that is appropriate for age, genetic background, and environment, as well as maintaining adequate hydration
- Stress and Resilience � Reducing stress levels and managing existing stress
- Relationships and Networks � Developing and maintaining healthy relationships and social networks while reducing the impact of noxious relationships
Personalized (Individualized) Medicine: Personalized medicine can be described as the effort to define and strengthen the art of individualizing health care by integrating the interpretation of patient data (medical history, family history, signs, and symptoms) with emerging ��omic� technologies�nutritional genomics, pharmacogenomics, proteomics, and metabolomics. It is also defined as medicine that treats each patient as a unique individual and takes into account the totality of personal history, family history, environment and lifestyle, physical presentation, genetic background, and mind/body/spirit. Interventions are tailored to each patient and adjusted based on the patient�s individualized response.�
Precipitating Event: Similar to a trigger�a trigger, however, only provokes illness as long as the person is exposed to it (or for a short while afterward), while a precipitating event initiates a change in health status that persists long after the exposure ends
Prospective Medicine (aka: 4-P Medicine): A relatively new concept introduced in 2003, prospective medicine is a descriptive rather than a prescriptive term, encompassing �personalized, predictive, preventive, and participatory medicine.� Snyderman argues persuasively that a comprehensive system of care would address not only new technologies (e.g., identification of biomarkers, use of electronic and personalized health records), but also delivery systems, reimbursement mechanisms, and the needs of a variety of stakeholders (government, consumers, employers, insurers, and academic medicine). Prospective medicine does not claim to stake out new scientific or clinical territory; instead, it focuses on creating an innovative synthesis of technologies and models�particularly personalized medicine (the �-omics�) and systems biology�in order to �determine the risk for individuals to develop specific diseases, detect the disease�s earliest onset, and prevent or intervene early enough to provide maximum benefit.�
Proteomics: The large-scale study of proteins, particularly their structures and functions, how they’re modified, when and where they’re expressed, how they’re involved in metabolic pathways, and how they interact with one another. The proteome is the entire complement of proteins, including the modifications made to a particular set of proteins, produced by an organism or system. This will vary with time and distinct requirements, or stresses, that a cell or organism undergoes. As a result, proteomics is much more complicated than genomics: an organism’s genome is more or less constant, while the proteome differs from cell to cell and from time to time.�
PURE: A heuristic mnemonic for assessment and treatment of toxicity-related disorders. Steps to consider when assessing and treating patients with toxic exposures include:
- Pattern Recognition � Recognize common patterns of toxicity signs and symptoms, including those associated with neurodevelopmental toxicity, immunotoxicity, mitochondrial toxicity, and endocrine toxicity
- Undersupported/Overexposed � Examine the patient�s environment and lifestyle to determine what might be lacking and what there might be too much of
- Reduce Toxin Exposure � Design a strategy for the patient to avoid continued toxin exposure
- Ensure a Safe Detox � Support the patient during detoxification by ensuring adequate nutrients to aid in the detoxification and biotransformation process and by recommending lifestyle changes that increase the safety and efficacy of detox programs.�
PTSD: A heuristic for general treatment of hormone-related disorders. Factors to be considered include:
- Production � Production/synthesis and secretion of the hormone
- What are the building blocks of thyroid hormone and cortisol?
- What affects the secretion of insulin?
- What are the building blocks of serotonin?
- What affects synthesis-inflammation of the gland (as in autoimmune thyroiditis)?
- Transport � Transport/conversion/distribution/ interaction with other hormones
- Do the levels of insulin impact the levels of E or T?
- Does a hormone�s transport from its gland of origin to the target gland impact its effectiveness or toxicity?
- Can we influence the level of free hormone?
- Is the hormone transformed (T4 to T3 or RT3) and can we modulate that?
- Sensitivity � Cellular sensitivity to the hormone signal
- Are there nutritional or dietary factors that influence the cellular response to insulin, thyroid hormones, estrogens, etc.?
- Detoxification � Detoxification/excretion of the hormone. For example:
- How is estradiol metabolized in the process of biotransformation?
- Can we alter it?
- What can we do to affect the binding to and excretion of estrogens?
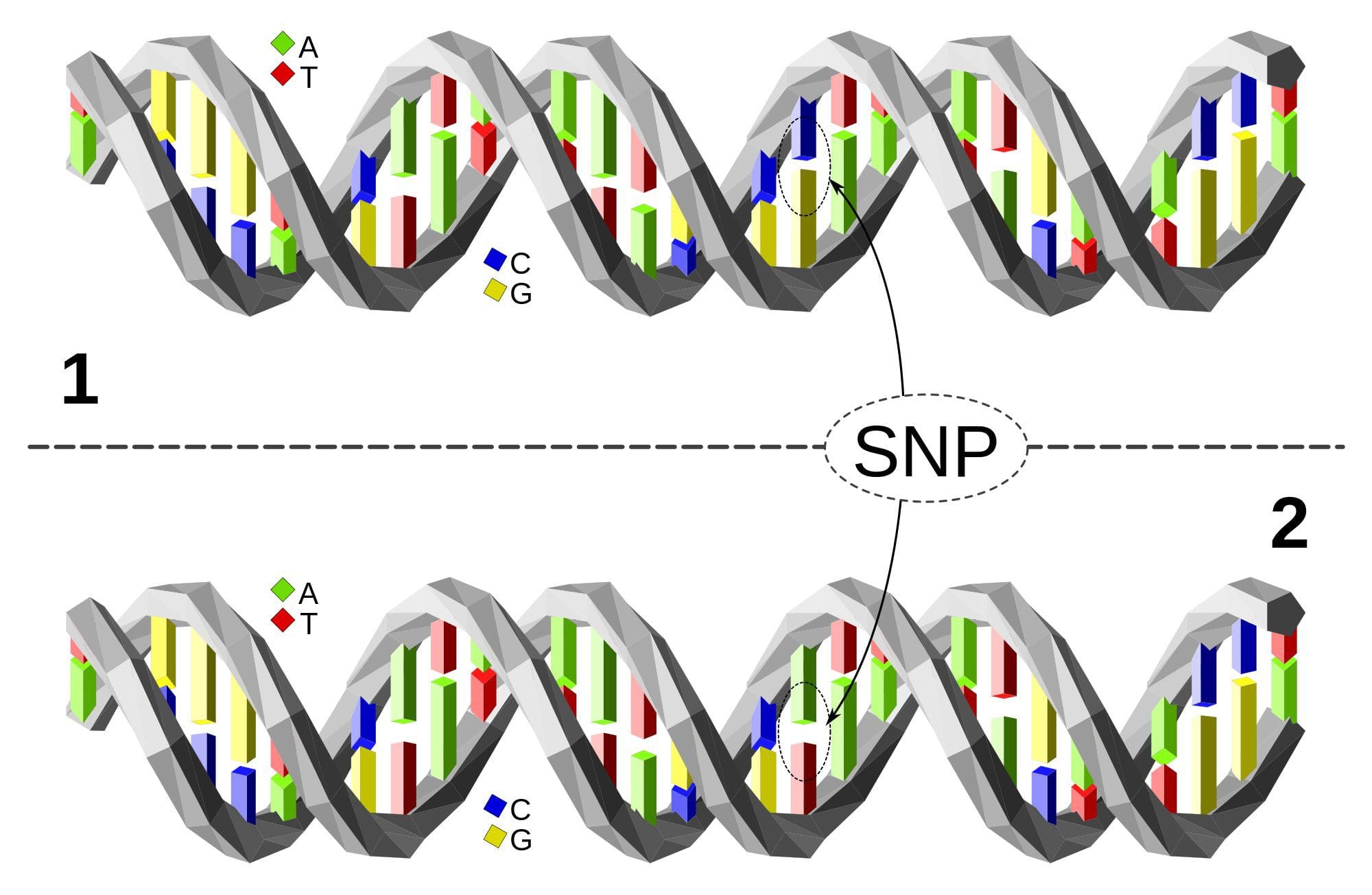 Single Nucleotide Polymorphism or SNP (pronounced �snip�) is a DNA sequence variation occurring when a single nucleotide�A, T, C, or G�in the genome differs between members of a species or between paired chromosomes in an individual. Almost all common SNPs have only two alleles. These genetic variations underlie differences in our susceptibility to, or protection from, several diseases. Variations in the DNA sequences of humans can affect how humans develop diseases. For example, a single base difference in the genes coding for apolipoprotein E is associated with a higher risk for Alzheimer’s disease. SNPs are also manifestations of genetic variations in the severity of illness, the way our body responds to treatments, and the individual response to pathogens, chemicals, drugs, vaccines, and other agents. They are thought to be key factors in applying the concept of personalized medicine.
Single Nucleotide Polymorphism or SNP (pronounced �snip�) is a DNA sequence variation occurring when a single nucleotide�A, T, C, or G�in the genome differs between members of a species or between paired chromosomes in an individual. Almost all common SNPs have only two alleles. These genetic variations underlie differences in our susceptibility to, or protection from, several diseases. Variations in the DNA sequences of humans can affect how humans develop diseases. For example, a single base difference in the genes coding for apolipoprotein E is associated with a higher risk for Alzheimer’s disease. SNPs are also manifestations of genetic variations in the severity of illness, the way our body responds to treatments, and the individual response to pathogens, chemicals, drugs, vaccines, and other agents. They are thought to be key factors in applying the concept of personalized medicine.
Relative Risk: A measure of the strength of the relationship between risk factors and a condition. For example, one could compare the risk of developing cancer in persons with a certain exposure or trait to the risk in persons who do not have this characteristic. Male smokers are about 23 times more likely to develop lung cancer than nonsmokers, so their relative risk is 23. Most relative risks are not this large. For example, women who have a first-degree relative (mother, sister, or daughter) with a history of breast cancer have about twice the risk of developing breast cancer compared to women who do not have this family history.�
Systems Biology: Although there is not yet a universally recognized definition of systems biology, the National Institute of General Medical Services (NIGMS) at NIH provides the following explanation: �A field that seeks to study the relationships and interactions between various parts of a biological system (metabolic pathways, organelles, cells, and organisms) and to integrate this information to understand how biological systems function.�
The 5Rs: A heuristic mnemonic for the five-step process used to normalize gastrointestinal function that is a core element of functional medicine:
- Remove � Removing the source of the imbalance (e.g., pathogens, allergic foods) is the critical first step.
- Replace � Next replace any factors that are missing (e.g., HCL, digestive enzymes)
- Reinoculate � Repopulate the gut with symbiotic bacteria (e.g., lactobacilli, bifidobacteria)
- Repair � Heal damaged gut membranes using, for example, glutamine, fiber, and butyrate
- Rebalance � Modify attitude, diet, and lifestyle of the patient to promote a healthier way of living
Three Legs of the Stool: A framework for practicing functional medicine that includes three parts:
- Retelling the patient�s story with ATMs (antecedents, triggers, and mediators): The clinician collects information from the patient through extensive interaction, then reflects the problem back to the patient in terms of antecedents, triggers, and mediators
- Organizing the clinical imbalances: The clinician organizes the clinical imbalances in the organizing physiological systems and lists them on the Functional Medicine Matrix.
- Personalized lifestyle factors: The clinician assesses each patient�s environment and lifestyle, and partners with patients to help them develop, adopt, and maintain appropriate personalized health-promoting behaviors.�
Timeline: A tool that allows clinicians to visualize a patient�s story chronologically by organizing important life events and health issues from pre-conception to the present.
 Triage Theory: Linus Pauling Award winner Bruce Ames� theory that DNA damage and late onset disease are consequences of a �triage allocation mechanism� developed during evolution to cope with periods of micronutrient shortage. When micronutrients (vitamins and minerals) are scarce, they are consumed for short-term survival at the expense of long-term survival. In 2009, Children�s Hospital and Research Center Oakland concluded that triage theory explains how diseases associated with aging like cancer, heart disease, and dementia (and the pace of aging itself) may be unintended consequences of mechanisms developed during evolution to protect against episodic vitamin/mineral shortages.
Triage Theory: Linus Pauling Award winner Bruce Ames� theory that DNA damage and late onset disease are consequences of a �triage allocation mechanism� developed during evolution to cope with periods of micronutrient shortage. When micronutrients (vitamins and minerals) are scarce, they are consumed for short-term survival at the expense of long-term survival. In 2009, Children�s Hospital and Research Center Oakland concluded that triage theory explains how diseases associated with aging like cancer, heart disease, and dementia (and the pace of aging itself) may be unintended consequences of mechanisms developed during evolution to protect against episodic vitamin/mineral shortages.
Triggers: Triggers are discrete entities or events that provoke disease or its symptoms (e.g., microbes). Triggers are usually insufficient in and of themselves for disease formation, however, because the health of the host and the vigor of its response to a trigger are essential elements.
 Xenobiotics: Chemicals found in an organism that are not normally produced by or expected to be present in that organism. This may also include substances present in much higher concentrations than usual. The term xenobiotics is often applied to pollutants such as dioxins and polychlorinated biphenyls, because xenobiotics are understood as substances foreign to an entire biological system, i.e. artificial substances that did not exist in nature before their synthesis by humans. Exposure to several types of xenobiotics has been implicated in cancer risk.
Xenobiotics: Chemicals found in an organism that are not normally produced by or expected to be present in that organism. This may also include substances present in much higher concentrations than usual. The term xenobiotics is often applied to pollutants such as dioxins and polychlorinated biphenyls, because xenobiotics are understood as substances foreign to an entire biological system, i.e. artificial substances that did not exist in nature before their synthesis by humans. Exposure to several types of xenobiotics has been implicated in cancer risk.





 Chronic undernutrition is characterized by a progressive reduction of the�fat-free mass (FFM) and fat mass (FM)�and �which has deleterious consequences on health. Undernutrition is insufficiently screened and treated in hospitalized or at-risk patients despite its high prevalence and negative impact on mortality, morbidity, length of stay (LOS), quality of life, and costs [1�4]. The risk of underestimating hospital undernutrition is likely to worsen in the next decades because of the increasing prevalence of overweight, obesity, and chronic diseases and the increased number of elderly subjects. These clinical conditions are associated with FFM loss (sarcopenia). Therefore, an increased number of patients with FFM loss and sarcopenic obesity will be seen in the future.
Chronic undernutrition is characterized by a progressive reduction of the�fat-free mass (FFM) and fat mass (FM)�and �which has deleterious consequences on health. Undernutrition is insufficiently screened and treated in hospitalized or at-risk patients despite its high prevalence and negative impact on mortality, morbidity, length of stay (LOS), quality of life, and costs [1�4]. The risk of underestimating hospital undernutrition is likely to worsen in the next decades because of the increasing prevalence of overweight, obesity, and chronic diseases and the increased number of elderly subjects. These clinical conditions are associated with FFM loss (sarcopenia). Therefore, an increased number of patients with FFM loss and sarcopenic obesity will be seen in the future.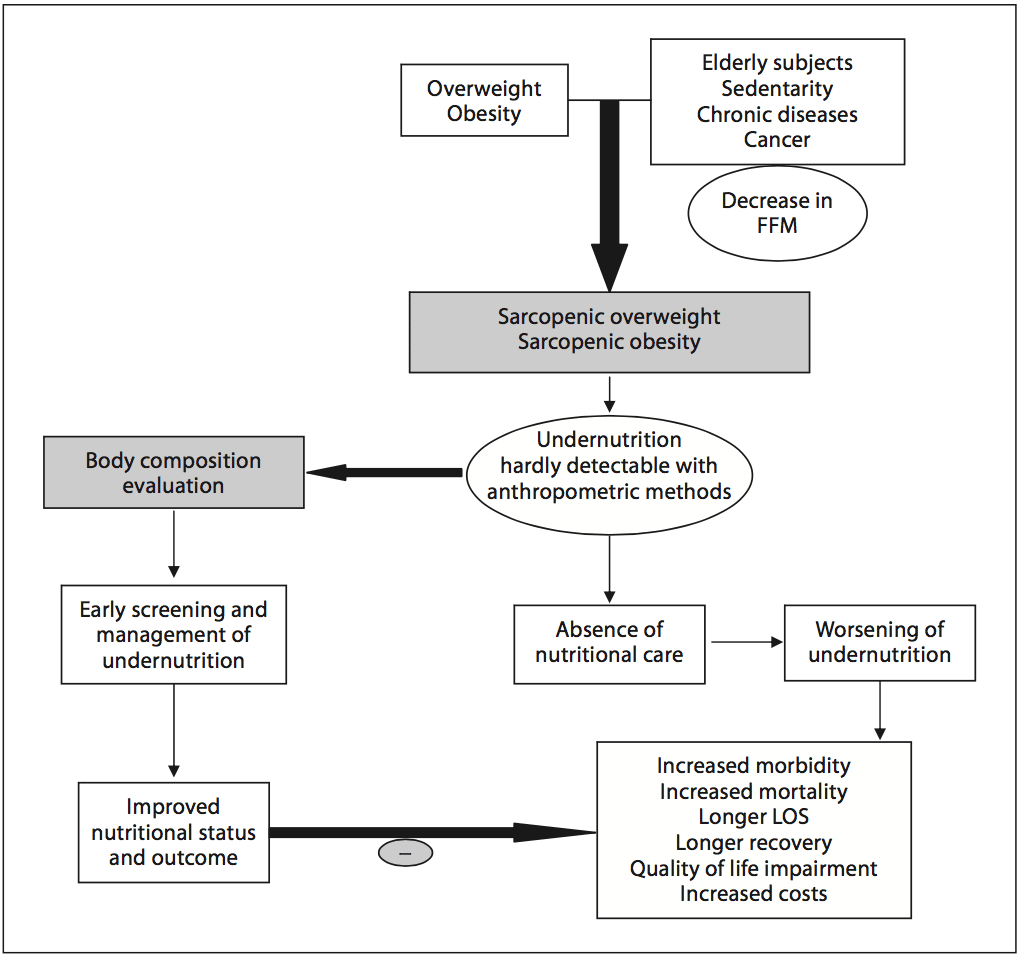
 Academic societies encourage systematic screening of undernutrition at hospital admission and during the hospital stay [14]. The detection of undernutrition is generally based on measurements of weight and height, calculations of BMI, and the percentage of weight loss. Nevertheless, screening of undernutrition is infrequent in hospitalized or nutritionally at-risk ambulatory patients. For example, in France, surveys performed by the French Health Authority [15] indicate that: (i) weight alone, (ii) weight with BMI or percentage of weight loss, and (iii) weight, BMI,�and percentage of weight loss are reported in only 55, 30, and 8% of the hospitalized patients� records, respectively. Several issues, which could be improved by specific educational programs, explain the lack of implementation of nutritional screening in hospitals (table 1). In addition, the accuracy of the clinical screening of undernutrition could be limited at hospital admission. Indeed, patients with undernutrition may have the same BMI as sex- and age- matched healthy controls but a significantly decreased FFM hidden by an expansion of the FM and the total body water which can be measured by bioelectrical impedance analysis (BIA) [13]. This example illustrates that body composition evaluation allows a more accurate identification of FFM loss than body weight loss or BMI decrease. The lack of sensitivity and specificity of weight, BMI, and percentage of weight loss argue for the need for other methods to evaluate the nutritional status.
Academic societies encourage systematic screening of undernutrition at hospital admission and during the hospital stay [14]. The detection of undernutrition is generally based on measurements of weight and height, calculations of BMI, and the percentage of weight loss. Nevertheless, screening of undernutrition is infrequent in hospitalized or nutritionally at-risk ambulatory patients. For example, in France, surveys performed by the French Health Authority [15] indicate that: (i) weight alone, (ii) weight with BMI or percentage of weight loss, and (iii) weight, BMI,�and percentage of weight loss are reported in only 55, 30, and 8% of the hospitalized patients� records, respectively. Several issues, which could be improved by specific educational programs, explain the lack of implementation of nutritional screening in hospitals (table 1). In addition, the accuracy of the clinical screening of undernutrition could be limited at hospital admission. Indeed, patients with undernutrition may have the same BMI as sex- and age- matched healthy controls but a significantly decreased FFM hidden by an expansion of the FM and the total body water which can be measured by bioelectrical impedance analysis (BIA) [13]. This example illustrates that body composition evaluation allows a more accurate identification of FFM loss than body weight loss or BMI decrease. The lack of sensitivity and specificity of weight, BMI, and percentage of weight loss argue for the need for other methods to evaluate the nutritional status. In 2008, twelve and thirty percent of the worldwide adult population was obese or overweight; this is two times higher than in 1980 [16]. The prevalence of overweight and obesity is also increasing in hospitalized patients. A 10-year comparative survey performed in a European hospital showed an increase in patients� BMI, together with a shorter LOS [17]. The BMI increase masks undernutrition and FFM loss at hospital admission. The increased prevalence of obesity in an aging population has led to the recognition of a new nutritional entity: �sarcopenic obesity� [18]. Sarcopenic obesity is characterized by increased FM and reduced FFM with a normal or high body weight. The emergence of the concept of sarcopenic obesity will increase the number of situations associated with a lack of sensitivity of the calculations of BMI and�body weight change for the early detection of FFM loss. This supports a larger use of body composition evaluation for the assessment and follow-up of nutritional status in clinical practice (fig. 1).
In 2008, twelve and thirty percent of the worldwide adult population was obese or overweight; this is two times higher than in 1980 [16]. The prevalence of overweight and obesity is also increasing in hospitalized patients. A 10-year comparative survey performed in a European hospital showed an increase in patients� BMI, together with a shorter LOS [17]. The BMI increase masks undernutrition and FFM loss at hospital admission. The increased prevalence of obesity in an aging population has led to the recognition of a new nutritional entity: �sarcopenic obesity� [18]. Sarcopenic obesity is characterized by increased FM and reduced FFM with a normal or high body weight. The emergence of the concept of sarcopenic obesity will increase the number of situations associated with a lack of sensitivity of the calculations of BMI and�body weight change for the early detection of FFM loss. This supports a larger use of body composition evaluation for the assessment and follow-up of nutritional status in clinical practice (fig. 1).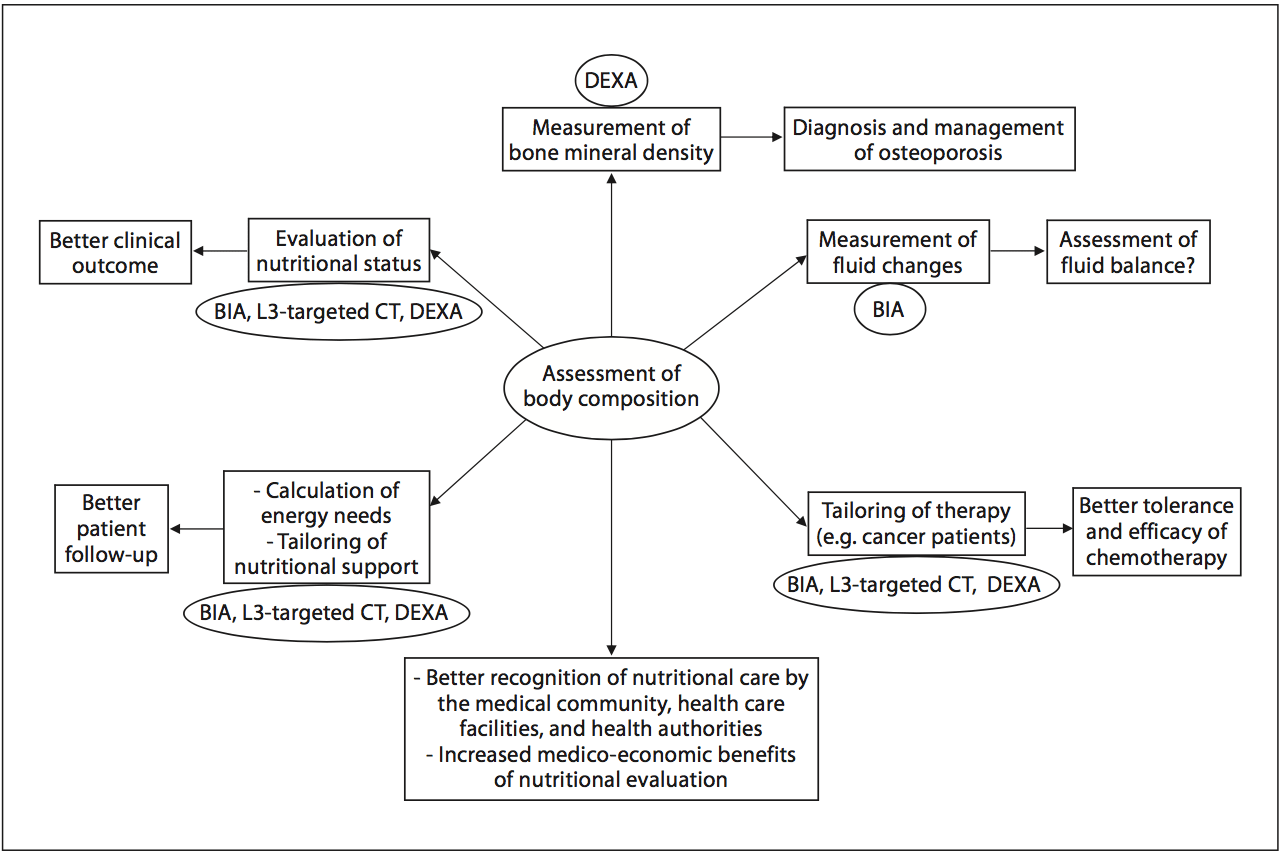 Fig. 2. Current and potential applications of body composition evaluation in clinical practice. The applications are indicated in the boxes, and the body composition methods that could be used for each application are indicated inside the circles. The most used application of body composition evaluation is the measurement of bone mineral density by DEXA for the diagnosis and management of osteoporosis. Although a low FFM is associated with worse clinical outcomes, FFM evaluation is not yet implemented enough in clinical practice. However, by allowing early detection of undernutrition, body composition evaluation could improve the clinical outcome. Body composition evaluation could also be used to follow up nutritional status, calculate energy needs, tailor nutritional support, and assess fluid changes during perioperative period and renal insufficiency. Recent evidence indicates that�a low FFM is associated with a higher toxicity of some chemo- therapy drugs in cancer patients. Thus, by allowing tailoring of the chemotherapy doses to the FFM in cancer patients, body com- position evaluation should improve the tolerance and the efficacy of chemotherapy. BIA, L3-targeted CT, and DEXA could be used for the assessment of nutritional status, the calculation of energy needs, and the tailoring of nutritional support and therapy. Further studies are warranted to validate BIA as an accurate method for fluid balance measurement. By integrating body composition evaluation into the management of different clinical conditions, all of these potential applications would lead to a better recognition of nutritional care by the medical community, the health care facilities, and the health authorities, as well as to an increase in the medico-economic benefits of the nutritional evaluation.
Fig. 2. Current and potential applications of body composition evaluation in clinical practice. The applications are indicated in the boxes, and the body composition methods that could be used for each application are indicated inside the circles. The most used application of body composition evaluation is the measurement of bone mineral density by DEXA for the diagnosis and management of osteoporosis. Although a low FFM is associated with worse clinical outcomes, FFM evaluation is not yet implemented enough in clinical practice. However, by allowing early detection of undernutrition, body composition evaluation could improve the clinical outcome. Body composition evaluation could also be used to follow up nutritional status, calculate energy needs, tailor nutritional support, and assess fluid changes during perioperative period and renal insufficiency. Recent evidence indicates that�a low FFM is associated with a higher toxicity of some chemo- therapy drugs in cancer patients. Thus, by allowing tailoring of the chemotherapy doses to the FFM in cancer patients, body com- position evaluation should improve the tolerance and the efficacy of chemotherapy. BIA, L3-targeted CT, and DEXA could be used for the assessment of nutritional status, the calculation of energy needs, and the tailoring of nutritional support and therapy. Further studies are warranted to validate BIA as an accurate method for fluid balance measurement. By integrating body composition evaluation into the management of different clinical conditions, all of these potential applications would lead to a better recognition of nutritional care by the medical community, the health care facilities, and the health authorities, as well as to an increase in the medico-economic benefits of the nutritional evaluation.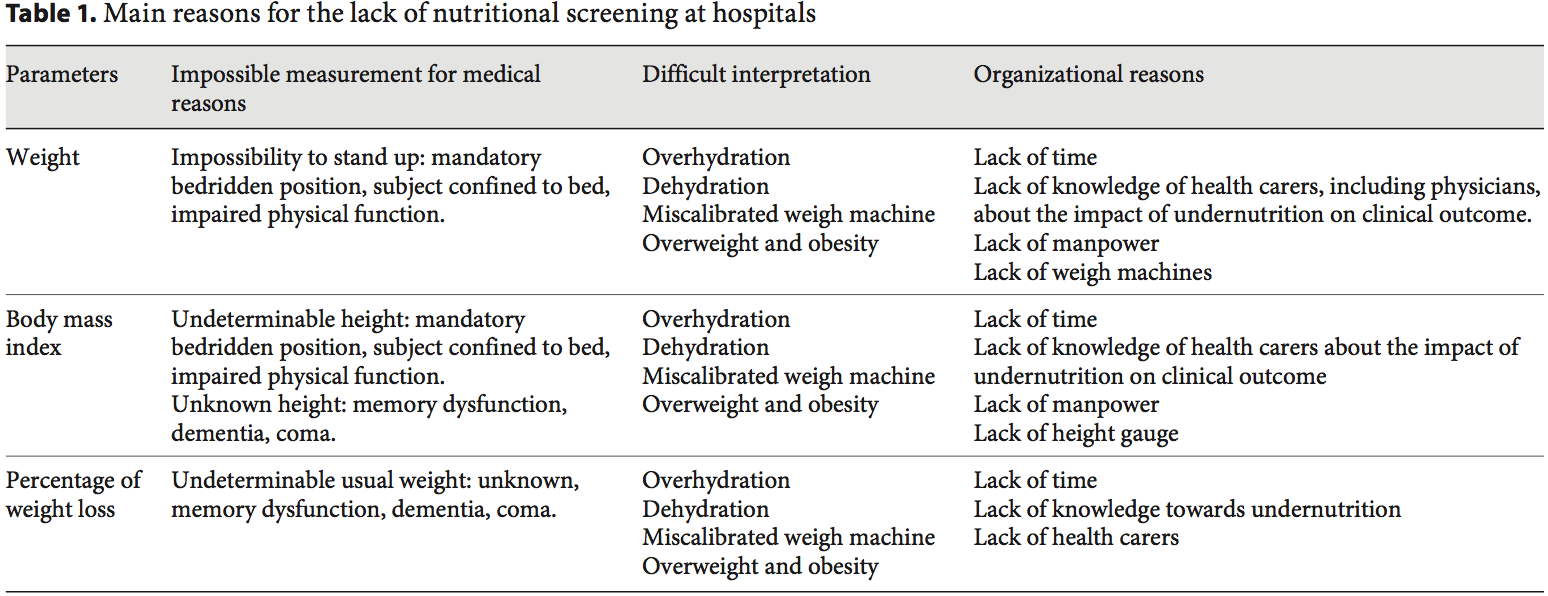
 Body Composition Techniques For FFM Measurement
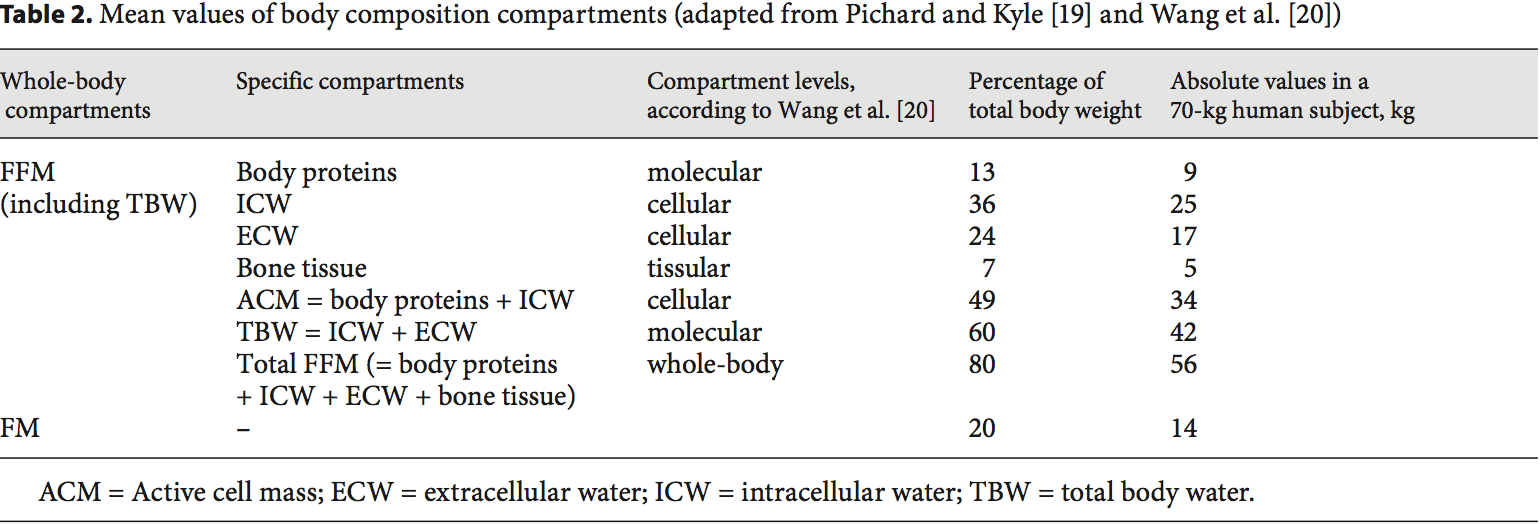
Body Composition Techniques For FFM Measurement Body composition evaluation allows measurement of the major body compartments: FFM (including bone mineral tissue), FM, and total body water. Table 2 shows indicative values of the body composition of a healthy subject weighing 70 kg. In several clinical situations, i.e. hospital admission, chronic obstructive pulmonary dis- ease (COPD) [21�23], dialysis [24�26], chronic heart failure [27], amyotrophic lateral sclerosis [28], cancer [5, 29], liver transplantation [30], nursing home residence [31], and Alzheimer�s disease [32], changes in body compartments are detected with the techniques of body composition evaluation. At hospital admission, body composition evaluation could be used for the detection of FFM loss and undernutrition. Indeed, FFM and the FFM index (FFMI) [FFM (kg)/height (m2)] measured by BIA are significantly lower in hospitalized patients (n = 995) than in age-, height-, and sex-matched controls (n = 995) [3]. Conversely, clinical tools of nutritional status assessment, such as BMI, subjective global assessment, or mini-nutritional assessment, are not accurate enough to estimate FFM loss and nutritional status [30, 32�34]. In 441 patients with non-small cell lung cancer, FFM loss deter- mined by computerized tomography (CT) was observed in each BMI category [7], and in young adults with all�types of cancer, an increase in FM together with a de- crease in FFM were reported [29]. These findings reveal the lack of sensitivity of BMI to detect FFM loss. More- over, the FFMI is a more sensitive determinant of LOS than a weight loss over 10% or a BMI below 20 [3]. In COPD, the assessment of FFM by BIA is a more sensitive method to detect undernutrition than anthropometry [33, 35]. BIA is also more accurate at assessing nutrition- al status in children with severe neurologic impairment than the measurement of skin fold thickness [36].
Body composition evaluation allows measurement of the major body compartments: FFM (including bone mineral tissue), FM, and total body water. Table 2 shows indicative values of the body composition of a healthy subject weighing 70 kg. In several clinical situations, i.e. hospital admission, chronic obstructive pulmonary dis- ease (COPD) [21�23], dialysis [24�26], chronic heart failure [27], amyotrophic lateral sclerosis [28], cancer [5, 29], liver transplantation [30], nursing home residence [31], and Alzheimer�s disease [32], changes in body compartments are detected with the techniques of body composition evaluation. At hospital admission, body composition evaluation could be used for the detection of FFM loss and undernutrition. Indeed, FFM and the FFM index (FFMI) [FFM (kg)/height (m2)] measured by BIA are significantly lower in hospitalized patients (n = 995) than in age-, height-, and sex-matched controls (n = 995) [3]. Conversely, clinical tools of nutritional status assessment, such as BMI, subjective global assessment, or mini-nutritional assessment, are not accurate enough to estimate FFM loss and nutritional status [30, 32�34]. In 441 patients with non-small cell lung cancer, FFM loss deter- mined by computerized tomography (CT) was observed in each BMI category [7], and in young adults with all�types of cancer, an increase in FM together with a de- crease in FFM were reported [29]. These findings reveal the lack of sensitivity of BMI to detect FFM loss. More- over, the FFMI is a more sensitive determinant of LOS than a weight loss over 10% or a BMI below 20 [3]. In COPD, the assessment of FFM by BIA is a more sensitive method to detect undernutrition than anthropometry [33, 35]. BIA is also more accurate at assessing nutrition- al status in children with severe neurologic impairment than the measurement of skin fold thickness [36]. FFM loss is correlated with survival in different clinical settings [5, 21�28, 37]. In patients with amyotrophic lateral sclerosis, an FM increase, but not an FFM in- crease, measured by BIA, was correlated with survival during the course of the disease [28]. The relation between body composition and mortality has not yet been demonstrated in the intensive care unit. The relation between body composition and mortality has been demonstrated with anthropometric methods, BIA, and CT. Measurement of the mid-arm muscle circumference is an easy tool to diagnose sarcopenia [38]. The mid-arm muscle circumference has been shown to be correlated with survival in patients with cirrhosis [39, 40], HIV infection [41], and COPD in a stronger way than BMI [42]. The relation between FFM loss and mortality has been extensively shown with BIA [21�28, 31, 37], which is the most used method. Recently, very interesting data suggest that CT could evaluate the disease prognosis in relation to muscle wasting. In obese cancer patients, sarcopenia as assessed by CT measurement of the total skeletal muscle cross-sectional area is an independent predictor of the survival of patients with bronchopulmonary [5, 7], gastrointestinal [5], and pancreatic cancers [6]. FFM assessed by measurement of the mid-thigh muscle cross- sectional area by CT is also predictive of mortality in COPD patients with severe chronic respiratory insufficiency [43]. In addition to mortality, a low FFMI at hospital admission is significantly associated with an in- creased LOS [3, 44]. A bicentric controlled population study performed in 1,717 hospitalized patients indicates that both loss of FFM and excess of FM negatively affect the LOS [44]. Patients with sarcopenic obesity are most at risk of increased LOS. This study also found that ex- cess FM reduces the sensitivity of BMI to detect nutritional depletion [44]. Together with the observation that the BMI of hospitalized patients has increased during the last decade [17], these findings suggest that FFM and�FFMI measurement should be used to evaluate nutritional status in hospitalized patients.
FFM loss is correlated with survival in different clinical settings [5, 21�28, 37]. In patients with amyotrophic lateral sclerosis, an FM increase, but not an FFM in- crease, measured by BIA, was correlated with survival during the course of the disease [28]. The relation between body composition and mortality has not yet been demonstrated in the intensive care unit. The relation between body composition and mortality has been demonstrated with anthropometric methods, BIA, and CT. Measurement of the mid-arm muscle circumference is an easy tool to diagnose sarcopenia [38]. The mid-arm muscle circumference has been shown to be correlated with survival in patients with cirrhosis [39, 40], HIV infection [41], and COPD in a stronger way than BMI [42]. The relation between FFM loss and mortality has been extensively shown with BIA [21�28, 31, 37], which is the most used method. Recently, very interesting data suggest that CT could evaluate the disease prognosis in relation to muscle wasting. In obese cancer patients, sarcopenia as assessed by CT measurement of the total skeletal muscle cross-sectional area is an independent predictor of the survival of patients with bronchopulmonary [5, 7], gastrointestinal [5], and pancreatic cancers [6]. FFM assessed by measurement of the mid-thigh muscle cross- sectional area by CT is also predictive of mortality in COPD patients with severe chronic respiratory insufficiency [43]. In addition to mortality, a low FFMI at hospital admission is significantly associated with an in- creased LOS [3, 44]. A bicentric controlled population study performed in 1,717 hospitalized patients indicates that both loss of FFM and excess of FM negatively affect the LOS [44]. Patients with sarcopenic obesity are most at risk of increased LOS. This study also found that ex- cess FM reduces the sensitivity of BMI to detect nutritional depletion [44]. Together with the observation that the BMI of hospitalized patients has increased during the last decade [17], these findings suggest that FFM and�FFMI measurement should be used to evaluate nutritional status in hospitalized patients. Numerous methods of body composition evaluation have been developed: anthropometry, including the 4-skinfold method [58], hydrodensitometry [58], in vivo neutron activation analysis [59], anthropogammametry from total body potassium-40 [60], nuclear magnetic resonance [61], dual-energy X-ray absorptiometry (DEXA) [62, 63], BIA [45, 64�66], and more recently CT [7, 43, 67]. DEXA, BIA, and CT appear to be the most convenient methods for clinical practice (fig. 2), while the other methods are reserved for scientific use.
Numerous methods of body composition evaluation have been developed: anthropometry, including the 4-skinfold method [58], hydrodensitometry [58], in vivo neutron activation analysis [59], anthropogammametry from total body potassium-40 [60], nuclear magnetic resonance [61], dual-energy X-ray absorptiometry (DEXA) [62, 63], BIA [45, 64�66], and more recently CT [7, 43, 67]. DEXA, BIA, and CT appear to be the most convenient methods for clinical practice (fig. 2), while the other methods are reserved for scientific use. The evaluation of FFM could be used for the calculation of energy needs, thus allowing the optimization of nutritional intakes according to nutritional needs. This could be of great interest in specific situations, such as severe neurologic disability, overweight, and obesity. In 61 children with severe neurologic impairment and intellectual disability, an equation integrating body composition had good agreement with the doubly labeled water method. It gave a better estimation of energy expenditure than did the Schofield predictive equation [36]. However, in 9 anorexia nervosa patients with a mean BMI of 13.7, pre- diction formulas of resting energy expenditure including FFM did not allow accurate prediction of the resting energy expenditure measured by indirect calorimetry [76]. In overweight or obese patients, the muscle catabolism in response to inflammation was the same as that observed�in patients with normal BMI. Indeed, despite a higher BMI, the FFM of overweight or obese individuals is similar (or slightly increased) to that of patients with normal BMI. Thus, the use of actual weight for the assessment of the energy needs of obese patients would result in over- feeding and its related complications. Therefore, the ex- perts recommend the use of indirect calorimetry or calculation of the energy needs of overweight or obese patients as follows: 15 kcal/kg actual weight/day or 20�25 kcal/kg ideal weight/day [77, 78], although these predictive formulas could be inaccurate in some clinical conditions [79]. In a US prospective study conducted in 33 ICU medical and surgical ventilated ICU patients, daily measurement of the active cell mass (table 2) by BIA was used to assess the adequacy between energy/protein intakes and needs. In that study, nutritional support with 30 kcal/ kg actual body weight/day energy and 1.5 g/kg/day protein allowed stabilization of the active cell mass [75]. Thus, follow-up of FFM by BIA could help optimize nutritional intakes when indirect calorimetry cannot be performed.
The evaluation of FFM could be used for the calculation of energy needs, thus allowing the optimization of nutritional intakes according to nutritional needs. This could be of great interest in specific situations, such as severe neurologic disability, overweight, and obesity. In 61 children with severe neurologic impairment and intellectual disability, an equation integrating body composition had good agreement with the doubly labeled water method. It gave a better estimation of energy expenditure than did the Schofield predictive equation [36]. However, in 9 anorexia nervosa patients with a mean BMI of 13.7, pre- diction formulas of resting energy expenditure including FFM did not allow accurate prediction of the resting energy expenditure measured by indirect calorimetry [76]. In overweight or obese patients, the muscle catabolism in response to inflammation was the same as that observed�in patients with normal BMI. Indeed, despite a higher BMI, the FFM of overweight or obese individuals is similar (or slightly increased) to that of patients with normal BMI. Thus, the use of actual weight for the assessment of the energy needs of obese patients would result in over- feeding and its related complications. Therefore, the ex- perts recommend the use of indirect calorimetry or calculation of the energy needs of overweight or obese patients as follows: 15 kcal/kg actual weight/day or 20�25 kcal/kg ideal weight/day [77, 78], although these predictive formulas could be inaccurate in some clinical conditions [79]. In a US prospective study conducted in 33 ICU medical and surgical ventilated ICU patients, daily measurement of the active cell mass (table 2) by BIA was used to assess the adequacy between energy/protein intakes and needs. In that study, nutritional support with 30 kcal/ kg actual body weight/day energy and 1.5 g/kg/day protein allowed stabilization of the active cell mass [75]. Thus, follow-up of FFM by BIA could help optimize nutritional intakes when indirect calorimetry cannot be performed. Body composition evaluation allows a qualitative assessment of body weight variations. The evaluation of body composition may help to document the efficiency of nutritional support during a patient�s follow-up of numerous clinical conditions, such as surgery [59], anorexia nervosa [76, 80], hematopoietic stem cell transplantation [81], COPD [82], ICU [83], lung transplantation [84], ulcerative colitis [59], Crohn�s disease [85], cancer [86, 87], HIV/AIDS [88], and acute stroke in elderly patients [89]. Body composition evaluation could be used for the follow-up of healthy elderly subjects [90]. Body composition evaluation allows characterization of the increase in body mass in terms of FFM and FM [81, 91]. After hematopoietic stem cell transplantation, the increase in BMI is the result of the increase in FM, but not of the increase in FFM [81]. Also, during recovery after an acute illness, weight gain 6 months after ICU discharge could be mostly related to an increase in FM (+7 kg) while FFM only increased by 2 kg; DEXA and air displacement plethysmography were used to measure the FM and FFM [91]. These two examples suggest that body composition evaluation could be helpful to decide the modification and/or the renewal of nutritional support. By identifying the patients gaining weight but reporting no or insufficient FFM, body composition evaluation could contribute to influencing the medical decision of continuing nutrition- al support that would have been stopped in the absence of body composition evaluation.
Body composition evaluation allows a qualitative assessment of body weight variations. The evaluation of body composition may help to document the efficiency of nutritional support during a patient�s follow-up of numerous clinical conditions, such as surgery [59], anorexia nervosa [76, 80], hematopoietic stem cell transplantation [81], COPD [82], ICU [83], lung transplantation [84], ulcerative colitis [59], Crohn�s disease [85], cancer [86, 87], HIV/AIDS [88], and acute stroke in elderly patients [89]. Body composition evaluation could be used for the follow-up of healthy elderly subjects [90]. Body composition evaluation allows characterization of the increase in body mass in terms of FFM and FM [81, 91]. After hematopoietic stem cell transplantation, the increase in BMI is the result of the increase in FM, but not of the increase in FFM [81]. Also, during recovery after an acute illness, weight gain 6 months after ICU discharge could be mostly related to an increase in FM (+7 kg) while FFM only increased by 2 kg; DEXA and air displacement plethysmography were used to measure the FM and FFM [91]. These two examples suggest that body composition evaluation could be helpful to decide the modification and/or the renewal of nutritional support. By identifying the patients gaining weight but reporting no or insufficient FFM, body composition evaluation could contribute to influencing the medical decision of continuing nutrition- al support that would have been stopped in the absence of body composition evaluation. In clinical situations when weight and BMI do not reflect the FFM, the evaluation of body composition should be used to adapt drug doses to the FFM and/or FM absolute values in every patient. This point has been recently illustrated in oncology patients with sarcopenic obesity. FFM loss was determined by CT as described above. In cancer patients, some therapies could affect body com- position by inducing muscle wasting [92]. In patients with advanced renal cell carcinoma [92], sorafenib induces a significant 8% loss of skeletal muscular mass at 12 months. In turn, muscle wasting in patients with BMI less than 25 was significantly associated with sorafenib toxicity in patients with metastatic renal cancer [8]. In metastatic breast cancer patients receiving capecitabine treatment, and in patients with colorectal cancer receiving 5-fluorouracile, using the convention of dosing per unit of body surface area, FFM loss was the determinant of chemotherapy toxicity [9, 10] and time to tumor progression [10]. In colorectal cancer patients administered 5-fluoruracil, low FFM is a significant predictor of toxicity only in female patients [9]. The variation in toxicity between women and men may be partially explained by the fact that FFM was lower in females. Indeed, FFM rep- resents the distribution volume of most cytotoxic chemo- therapy drugs. In 2,115 cancer patients, the individual variations in FFM could change by up to three times the distribution volume of the chemotherapy drug per body area unit [5]. Thus, administering the same doses of chemotherapy drugs to a patient with a low FFM compared to a patient with a normal FFM would increase the risk of chemotherapy toxicity [5]. These data suggest that FFM loss could have a direct impact on the clinical outcome of cancer patients. Decreasing chemotherapy doses in case of FFM loss could contribute to improving cancer patients� prognosis through the improvement of the tolerance of chemotherapy. These findings justify the systematic evaluation of body composition in all cancer patients in order to detect FFM loss, tailor chemotherapy doses according to FFM values, and then improve the efficacy- tolerance and cost-efficiency ratios of the therapeutic strategies [93]. Body composition evaluation should also be used to tailor the doses of drugs which are calculated based on patients� weight, e.g. corticosteroids, immuno-suppressors (infliximab, azathioprine or methotrexate), or sedatives (propofol).
In clinical situations when weight and BMI do not reflect the FFM, the evaluation of body composition should be used to adapt drug doses to the FFM and/or FM absolute values in every patient. This point has been recently illustrated in oncology patients with sarcopenic obesity. FFM loss was determined by CT as described above. In cancer patients, some therapies could affect body com- position by inducing muscle wasting [92]. In patients with advanced renal cell carcinoma [92], sorafenib induces a significant 8% loss of skeletal muscular mass at 12 months. In turn, muscle wasting in patients with BMI less than 25 was significantly associated with sorafenib toxicity in patients with metastatic renal cancer [8]. In metastatic breast cancer patients receiving capecitabine treatment, and in patients with colorectal cancer receiving 5-fluorouracile, using the convention of dosing per unit of body surface area, FFM loss was the determinant of chemotherapy toxicity [9, 10] and time to tumor progression [10]. In colorectal cancer patients administered 5-fluoruracil, low FFM is a significant predictor of toxicity only in female patients [9]. The variation in toxicity between women and men may be partially explained by the fact that FFM was lower in females. Indeed, FFM rep- resents the distribution volume of most cytotoxic chemo- therapy drugs. In 2,115 cancer patients, the individual variations in FFM could change by up to three times the distribution volume of the chemotherapy drug per body area unit [5]. Thus, administering the same doses of chemotherapy drugs to a patient with a low FFM compared to a patient with a normal FFM would increase the risk of chemotherapy toxicity [5]. These data suggest that FFM loss could have a direct impact on the clinical outcome of cancer patients. Decreasing chemotherapy doses in case of FFM loss could contribute to improving cancer patients� prognosis through the improvement of the tolerance of chemotherapy. These findings justify the systematic evaluation of body composition in all cancer patients in order to detect FFM loss, tailor chemotherapy doses according to FFM values, and then improve the efficacy- tolerance and cost-efficiency ratios of the therapeutic strategies [93]. Body composition evaluation should also be used to tailor the doses of drugs which are calculated based on patients� weight, e.g. corticosteroids, immuno-suppressors (infliximab, azathioprine or methotrexate), or sedatives (propofol).
 The implementation of body composition evaluation in routine care presents a challenge for the next decades. Indeed the concomitant increases in elderly subjects and patients with chronic diseases and cancer, and in the prevalence of overweight and obesity in the population, will increase the number of patients nutritionally at risk or undernourished, particularly those with sarcopenic obesity. Body composition evaluation should be used to improve the screening of undernutrition in hospitalized patients. The results of body composition should be based on the same principle as BMI calculation, towards the systematic normalization for body height of FFM (FFMI) and FM [FM (kg)/height (m)2 = FM index] [94]. The results could be expressed according to previously de- scribed percentiles of healthy subjects [95, 96]. Body com- position evaluation should be performed at the different stages of the disease, during the course of treatments and the rehabilitation phase. Such repeated evaluations of body composition could allow assessment of the nutritional status, adjusting the calculation of energy needs as kilocalories/kilogram FFM, following the efficacy of
The implementation of body composition evaluation in routine care presents a challenge for the next decades. Indeed the concomitant increases in elderly subjects and patients with chronic diseases and cancer, and in the prevalence of overweight and obesity in the population, will increase the number of patients nutritionally at risk or undernourished, particularly those with sarcopenic obesity. Body composition evaluation should be used to improve the screening of undernutrition in hospitalized patients. The results of body composition should be based on the same principle as BMI calculation, towards the systematic normalization for body height of FFM (FFMI) and FM [FM (kg)/height (m)2 = FM index] [94]. The results could be expressed according to previously de- scribed percentiles of healthy subjects [95, 96]. Body com- position evaluation should be performed at the different stages of the disease, during the course of treatments and the rehabilitation phase. Such repeated evaluations of body composition could allow assessment of the nutritional status, adjusting the calculation of energy needs as kilocalories/kilogram FFM, following the efficacy of 







