
by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gastro Intestinal Health, Gut and Intestinal Health, Health, Nutrition, Wellness
Do you feel:
- A sense of fullness during and after meals?
- Do digestive problems subside with rest and relaxation?
- Diarrhea?
- Unpredictable abdominal swelling?
- Frequent bloating and distention after eating?
If you are experiencing any of these situations, then you might be experiencing problems with your digestive tract. Here are some ways to improve your digestion problems naturally.
Different factors can impact a person’s digestion and overall gut health. There are things that people have control like how much sleep they are getting while the other things that are not in a person’s control like genetics and family history. If a person is experiencing stomach problems, then it might be the poor lifestyle choices that may be hurting their gut. Having a well-balanced diet and regularly exercising is good, but those are just two of the many ways to regulate digestive health.
Here are some of the lifestyles that may negatively impact the body�s gut health:
- What a person is eating
- Mindful of mindless eating
- Exercise routine
- Daily hydration
- Sleep schedule
- Stress and anxiety levels
- Prescription and over the counter medications a person takes
- Bad habits like late-night eating or excessive alcohol or tobacco use
These factors can do bodily harm and can cause the development of chronic illnesses.
11 Ways To Improve Digestive Health
Even though these factors can negatively affect a person�s digestion tract and overall gut health, there are 11 ways to help improve the digestive tract naturally and be beneficial to not only the gut but to the body.
Eating More Colorful, Plant-based and Fiber-Rich Foods

Even though digestive issues can be challenging, avoiding certain foods and eating more plant-based and fiber-rich foods can help ease those uncomfortable symptoms. Quality nut and seeds, vegetables, whole grains, and legumes can protect against a lot of digestive disorders and promote a regular bowel movement. To avoid discomfort on the digestive tract, try avoiding certain foods that are tough on the stomach like fried, artificially processed, or acidic foods.
If a person is suffering from an upset stomach or been diagnosed with IBS (irritable bowel syndrome), they might want to consider adopting an anti-inflammatory rich diet to prevent inflammation in the gut.
Consider Meal Frequency and Sizing
When a person continually snacking or tend to have three big meals a day is known as a grazer. Grazing food may not be suitable for people due to being prone to constipation. These habits can impact the person’s digestive health, and recent clinical studies have been shown that intermittent fasting can be beneficial to gut health and the whole body.
Practicing Mindful Eating
Sometimes overeating and eating too quickly can often lead to unpleasant indigestion symptoms such as gas and bloating. Thankfully there is an inclusive practice known as mindful eating, and it has been studied to a practical approach to reducing indigestion in the gut. Research has shown that mindful eating can reduce symptoms of IBS and ulcerative colitis.
To practice eating mindfully, keep in mind the following:
- Turning off the tv and putting away the phones at mealtimes.
- Taking a moment and inhale after sitting down with the plate in front of the individual. Take notice of how it smells.
- You are taking in on how the food looks on the plate.
- Select each bite of consciously.
- Chew the bites of food slowly.
- Eat slowly.
- Take breaks, sip water, or have a quick chat in between each bite.
- Take in the taste, texture, and temperature of every bite.
- Take time to relax after finishing a meal.
Following these tricks and taking the time to relax and paying attention to the body before a meal may improve digestive symptoms such as indigestion and bloating.
Exercise Regularly

Exercise can help digestion. When people move their bodies on a day to day basis can affect their digestion. Since it is mostly due to its anti-inflammatory effects, exercise can have a very positive impact on the digestive system. Studies have shown that living a sedentary lifestyle can be damaging to the gut. Working out can help a person relieve their stress, enable them to maintain a healthy weight, strengthen abdominal muscles, and stimulate food to move through the large intestines.
According to research, aerobic exercises, like dancing or high interval workout classes, are particularly great by increasing the blood flow to the GI tract. Keep in mind that it is best to avoid this type of high impact exercise right after eating. If an individual has a sensitive stomach, resting for 30 minutes in between workouts and meals is the best option.
Staying Hydrated

Not drinking enough water is a common cause of constipation among adults and children, since lots of people often replace water with sugary alternatives. Studies have shown that people should aim to drink at least 1.5 to 2 liters of non-caffeinated beverages daily to prevent constipation, and if they exercise, they should be drinking more water.
They can also increase their water intake by eating fruits that have high water content, drinking herbal teas, and non-caffeinated beverages like flavored seltzer waters.
Trying to Get A Good Night Sleep
Not getting enough hours to sleep and poor quality sleep has been associated with several gastrointestinal diseases. Studies show that people who are sleep deprived are most likely to suffer from stomach pains, diarrhea, upset stomach, or even suffer from inflammatory bowel disease. So people need to get quality sleep as the main priority.
Practice Ways to Manage Stress
Stress can affect a person�s digestion and the gastrointestinal tract big time. When an individual is chronically stressed out, their body is continuously in a flight or fight mode. Being chronically stressed out can lead to several unpleasant digestive symptoms such as constipation, diarrhea, bloating, IBS, and stomach ulcers.
There are ways to relieve stress through stress management techniques like yoga, acupuncture, cognitive behavioral therapy, and meditation. Research shows that these techniques have been shown to improve symptoms in people with IBS drastically. Even taking the time to sit quietly and practicing breathing exercises for five minutes can help alleviate stress levels.
Cutting Back on Drinking Alcohol

Many individuals experience diarrhea and several other unpleasant symptoms after consuming alcohol. This is because alcohol can trigger some severe changes in the digestive system. Studies have mentioned that when the gastrointestinal tract comes in contact with alcohol, it becomes inflamed. This is because the intestines do not absorb water as efficiently, causing the overall digestion to speed up, and the good/harmful bacteria balance is thrown off.
Stop Smoking
Smoking can impact the entire body, including the gut. Studies have shown that smoking, chewing, and vaping tobacco has been linked to several common disorders in the digestive system, such as heartburn, peptic ulcers, and GERD (gastroesophageal reflux disease). Smoking can also worsen gastrointestinal symptoms in other conditions like Crohn’s disease. When a person quits smoking, it can quickly reverse some of the effects of smoking on the digestive system and can keep the symptom of some gastrointestinal diseases from becoming worse.
Consider Taking Supplements
Taking dietary supplements is a great way to make sure that the body is getting the nutrients it needs for proper digestion.
- Probiotics are excellent digestive supplements to alleviate and improve symptoms of gas, to bloat, and stomach pains for people with IBS.
- Glutamine is an amino acid that supports gut health. Studies show that glutamine can reduce leaky gut in people who are sick.
- Zinc is a mineral that is essential for a healthy gut. When a person has a deficiency in zinc, it can lead to a variety of unpleasant digestive disorders. So taking zinc supplements can be beneficial to reducing digestive problems.
Be Aware of Medication Interactions and Their Side Effects
The medication that a person is taking can cause stomach discomfort and make them prone to diarrhea or constipation. Conventional medication such as aspirin and other pain medicine have been studied to upset the lining of the stomach, causing damage to the intestinal permeability.
Conclusion
Practicing these 11 ways can be beneficial and provide improvement to a person’s digestive tract. When disruptive factors disrupt the digestive tract, it can lead the body to have inflammation, leaky gut, and digestive problems. Some products are specialized to support the gastrointestinal tract and provide support to the body’s metabolism to make sure the body is functioning correctly.
The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues or functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or disorders of the musculoskeletal system. Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900.
References:
Ali, Tauseef, et al. �Sleep, Immunity and Inflammation in Gastrointestinal Disorders.� World Journal of Gastroenterology, Baishideng Publishing Group Co., Limited, 28 Dec. 2013, www.ncbi.nlm.nih.gov/pmc/articles/PMC3882397/.
Bilski, Jan, et al. �Can Exercise Affect the Course of Inflammatory Bowel Disease? Experimental and Clinical Evidence.� Pharmacological Reports: PR, US National Library of Medicine, Aug. 2016, www.ncbi.nlm.nih.gov/pubmed/27255494.
Bischoff, Stephan C. �’Gut Health’: a New Objective in Medicine?� BMC Medicine, BioMed Central, 14 Mar. 2011, www.ncbi.nlm.nih.gov/pmc/articles/PMC3065426/.
Catterson, James H, et al. �Short-Term, Intermittent Fasting Induces Long-Lasting Gut Health and TOR-Independent Lifespan Extension.� Current Biology: CB, Cell Press, 4 June, 2018, www.ncbi.nlm.nih.gov/pmc/articles/PMC5988561/.
Chiba, Mitsuro, et al. �Recommendation of Plant-Based Diets for Inflammatory Bowel Disease.� Translational Pediatrics, AME Publishing Company, Jan. 2019, www.ncbi.nlm.nih.gov/pmc/articles/PMC6382506/.
Didari, Tina, et al. �Effectiveness of Probiotics in Irritable Bowel Syndrome: Updated Systematic Review with Meta-Analysis.� World Journal of Gastroenterology, Baishideng Publishing Group Inc, 14 Mar. 2015, www.ncbi.nlm.nih.gov/pubmed/25780308.
Konturek, Peter C, et al. “Stress and the Gut: Pathophysiology, Clinical Consequences, Diagnostic Approach, and Treatment Options.” Journal of Physiology and Pharmacology: an Official Journal of the Polish Physiological Society, US National Library of Medicine, Dec. 2011, www.ncbi.nlm.nih.gov/pubmed/22314561.
Kristeller, Jean L, and Kevin D Jordan. �Mindful Eating: Connecting With the Wise Self, the Spiritual Self.� Frontiers in Psychology, Frontiers Media SA, 14 Aug. 2018, www.ncbi.nlm.nih.gov/pmc/articles/PMC6102380/.
Lakatos, Peter Laszlo. �Environmental Factors Affecting Inflammatory Bowel Disease: Have We Made Progress?� Digestive Diseases (Basel, Switzerland), US National Library of Medicine, 2009, www.ncbi.nlm.nih.gov/pubmed/19786744.
Miller, Carla K, et al. �Comparative Effectiveness of a Mindful Eating Intervention to a Diabetes Self-Management Intervention among Adults with Type 2 Diabetes: a Pilot Study.� Journal of the Academy of Nutrition and Dietetics, US National Library of Medicine, Nov. 2012, www.ncbi.nlm.nih.gov/pmc/articles/PMC3485681/.
Mottaghi, Azadeh, et al. �Efficacy of Glutamine-Enriched Enteral Feeding Formulae in Critically Ill Patients: a Systematic Review and Meta-Analysis of Randomized Controlled Trials.� Asia Pacific Journal of Clinical Nutrition, US National Library of Medicine, 2016, www.ncbi.nlm.nih.gov/pubmed/27440684.
Oettl�, G J. �Effect of Moderate Exercise on Bowel Habit.� Gut, US National Library of Medicine, Aug. 1991, www.ncbi.nlm.nih.gov/pubmed/1885077.
Philpott, HL, et al. “Drug-Induced Gastrointestinal Disorders.” Frontline Gastroenterology, BMJ Publishing Group, Jan. 2014, www.ncbi.nlm.nih.gov/pmc/articles/PMC5369702/.
Popkin, Barry M, et al. �Water, Hydration, and Health.� Nutrition Reviews, US National Library of Medicine, Aug. 2010, www.ncbi.nlm.nih.gov/pmc/articles/PMC2908954/.
Qin, Hong-Yan, et al. �Impact of Psychological Stress on Irritable Bowel Syndrome.� World Journal of Gastroenterology, Baishideng Publishing Group Inc, 21 Oct. 2014, www.ncbi.nlm.nih.gov/pmc/articles/PMC4202343/.
Skrovanek, Sonja, et al. �Zinc and Gastrointestinal Disease.� World Journal of Gastrointestinal Pathophysiology, Baishideng Publishing Group Inc, 15 Nov. 2014, www.ncbi.nlm.nih.gov/pubmed/25400994.
Unknown, Unknown. �11 Ways To Improve Digestion Problems Naturally.� Fullscript, 9 Sept. 2019, fullscript.com/blog/lifestyle-tips-for-digestive-health.
Unknown, Unknown. �Smoking and the Digestive System.� National Institute of Diabetes and Digestive and Kidney Diseases, US Department of Health and Human Services, 1 Sept. 2013, www.niddk.nih.gov/health-information/digestive-diseases/smoking-digestive-system.
Wong, Ming-Wun, et al. �Impact of Vegan Diets on Gut Microbiota: An Update on the Clinical Implications.� Ci Ji Yi Xue Za Zhi = Tzu-Chi Medical Journal, Medknow Publications & Media Pvt Ltd, 2018, www.ncbi.nlm.nih.gov/pmc/articles/PMC6172896/.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Chiropractic, Functional Medicine, Health, Health Coaching, Nutrition, Wellness
Health coaches are becoming more and more crucial as modern medicine continues to improve. Now more than ever, the health care field is progressing at high speeds and professionals do not always have the available time some patients desire. Here is where health coaches become involved. Basically, the position of a health coach was produced to fill the emptiness in several doctor offices. Many physicians contribute but do not have the time or resources to assist each individual and aid in constructing healthy habits on a day to day basis. However, health coaches are available to be a supportive mentor that assists and guides patients in making healthy lifestyle changes. Many patients who seek help to change their lifestyle are those suffering from some kind of chronic pain, headaches, or joint inflammation.
In the previous weeks, we have defined and explained what a health coach is and what they really do, as well as the first two steps a health coach might take with a patient. Throughout this article, the third and fourth steps will be broken down and analyzed.
Need a refresher? No problem!
Health Coaching in El Paso: Part 1 can be found by clicking here.�
Health Coaching in El Paso: Part 2 can be found by clicking here.�
Step 3: Building A Plan For Action
Once the health coach understands the values and goals of the patient, a plan for change can get mapped out. One thing that is unique about building a plan, is that the health coach encourages the patient to have a say in it and contribute to building the plan. The ways of medicine have changed, and this aspect is one of them. Before, many patients would sit silently as doctors instructed them on their new protocol. However, it has been shown that patients who build a plan of action with the practitioner, are more likely to comply and complete a program.
In addition to this, the perspective of the patient can help maintain expectations and keep the plan of action at a realistic timeline. The health coach will offer their suggestions during this process as well as their perspective. Often times, this will help the patient break down their overall goal, into smaller more specific goals or tasks.
As soon as the overall goals are broken down into specific tasks, the health coach will then map out the process to complete these tasks. It can be simple to overlook small steps when thinking of a bigger picture, so the health coach will provide tools to better help the patient understand.
An example of this would be for a patient who wants to lose weight. Mapping out these tasks will have an end result that looks similar to these:
� I will try a new fruit and vegetable every day this week and identify what I enjoy
� I will think of different, creative ways to work movement into my day, such as finding a walking trail in my neighborhood
� I will always keep a water bottle with me and refill it every two hours
� I will cook dinner two nights this week
� I will go for a walk after dinner every day this week
By providing the patients with these smaller tangible tasks, the patient now has “homework” in a sense to complete these throughout the week. The health coach will set a deadline with these tasks and check-in with the patient regularly to ensure they are on track.
Step 4: Tracking Progress And Results

Before progress can be tracked, the health coach will take into consideration the patient’s goal and determine how often the patient will need to come in for follow-ups. For many patients, a combination of follow up techniques are used. Health coaches understand that in-person is not always the most convenient and does not always fit into the patient’s schedule. If this is the scenario, health coaches work around that to create follow-ups by using some in-person visits, some phone conversations, or other virtual check-in meetings that are HIPAA compliant.
Often times, during a lifestyle change patients may become confused or discouraged. It is important to remember that this is normal and progress is not always a straight line up, but rather includes bumps along the way. In order to better help the patient, the health coach will provide them with a helpful “where to turn” guide.
As humans, at different times we require different types of support. The where to turn guide will be a supporting reminder of things to do to counteract these feelings when they arise. Items included in this guide will be ideas such as:
� Pursuing a hobby, like dancing or playing an instrument
� Getting out in nature
� Starting a mindfulness practice
� Making art, like drawing or writing
� Joining a community, religious, or spiritual group
In addition to these activities, the health coach will determine with the patient what kind of support (internal or external) is appropriate depending on the situation.
Lastly,� progress does not always look like a dip in the number on the scale. Progress can come in many different forms. In order to help the patient appreciate and realize all the progress they are making, a health coach will ask questions like:
1. How can you appreciate your progress?
2. How would you describe the benefits of your experience?
3. What�s been good about this experience?
4. How have you grown?
As mentioned earlier, a health coach is important to have as they help one realize all the steps it truly takes to be successful and reach their health goals. There are many critical steps that are easily overlooked when the big picture is on their minds. The final two steps that a health coach will work on with a patient is to help them visualize their best self and to create a plan for resiliency. These two topics will be discussed in the next article.
�Using a health coach to complete a lifestyle change is similar to the work of going to therapy. One must be willing to accept the tools and resources they are givien, and actually do the work provided or it will not produce results. If a patient is truly serious about completing a lifestyle change, using a health coach is an extremly beneifical resource! As one can see, they work with the patients to hammer down tasks and ideas that a patient might not have orignally thought of. By utilizing a health coach, the patient has a higher chance of reaching their goals. – Kenna Vaughn, Senior Health Coach
All information and resources for this post came from an Integrative Practioner article titled, “A Six-Step Approach To Health And Wellness Coaching: A Toolkit for Practice Implementation” and can be found by clicking here; as well as listed below in the proper bibliography.
*The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues or functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or disorders of the musculoskeletal system. Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900.
Bibliography:
American Psychological Association (2019). The Road to Resilience. Retrieved from: https://www.apa.org/helpcenter/road-resilience
Jonas, W. (2019). Empowering patients with chronic diseases to live healthier through health coaching: Integrative primary care case study. Samueli Integrative Health Programs.�Retrieved from: https://www.health.harvard.edu/staying-healthy/give-yourself-a-health-self-assessment
Miller, W. and Rose, G. (1991). Motivational Interviewing: Preparing People to Change Addictive Behavior. Guilford Publications.
Pecoraro, Wendy. �A Six-Step Approach to Health and Wellness Coaching: A Toolkit for Practice Implementation.� Official Media Integrative Practitioner, 17 Oct. 2019, www.integrativepractitioner.com/resources/e-books/a-six-step-approach-to-health-and-wellness-coaching-a-toolkit-for-practice-implementation.
Trzeciak, S. and Mazzarelli, A. (2019). Compassionomics. Studer Group. Virginia Polytechnic Institute and State University. The Stages of Change.Retrieved from: http://www.cpe.vt.edu/gttc/presentations/8eStagesofChange.pdf
Your Coach (2009). SMART goals.Retrieved from: https://www.yourcoach.be/en/coaching-tools/

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Health, Nutrition, Wellness
Do you feel:
- Aches, pains, and swelling throughout the body?
- Stomach pains, burning, or aching 1-4 hours after eating?
- Excessive belching, burping, or bloating?
- Inflammation in your stomach?
- Is gas immediately following a meal?
If you are experiencing any of these situations, then try these eating mushrooms for your immune system.
Mushrooms

Medicinal mushrooms have been traditionally used for centuries by protecting anyone against infectious diseases, and various cancers. The positive biological effects of mushrooms are due in part to the indirect action of stimulating the immune cells. These mushrooms have a long history of usages by supporting health, especially in early Chinese, Egyptian, Greek, Mexican, and Roman cultures. In fact in 1991, a 5,300-year-old mummy was discovered carrying polypore fungus, which exerts a purgative effect. It may have been used to treat the mummies’ intestinal parasites.
What Are the Benefits of Mushrooms?
Modern research has shown that medicinal mushrooms can provide a rich source of nutrients and bioactive compounds that are associated with a few health effects that primarily support the immune system. Mushrooms act as an anti-bacterial, immune system enhancer and cholesterol-lowering agents. Additionally, they are an essential source of bioactive compounds, and some mushroom extracts are used to promote human health as well as being found as dietary supplements.
Since medicinal mushrooms are edible macroscopic fungi that are visible to the naked eye and are used for their beneficial health properties. Fungi, which includes yeasts molds, and mushrooms, live on the dead matter that is found in soil, plants, animals, and other fungi. It is estimated that there are 14000 to 22000 known species of mushrooms worldwide and approximately 20 to 30 mushrooms that are cultivated edible species. Even though there approximately 15 species that are wild foraged for consumption, they can be part of functional foods or dietary supplements.
Mushrooms are a source of many nutrients, including fiber, protein, selenium, potassium, and vitamins, B1, B2, B12, C, D, and E. They also possess several bioactive components like alkaloids, flavonoids, terpenes, phenolic compounds, polyunsaturated fatty acids, and polysaccharides. Mushrooms have been studied for not only its immune-stimulating and prebiotic properties, but they notably contain ?- glucan, which is a polysaccharide that is commonly present in mushrooms.
Research has been examining the health effects of mushrooms and has identified approximately 130 possible therapeutic properties, including:
- Antibacterial
- Antidiabetic
- Antifungal
- Anti-inflammatory
- Antioxidants
- Antiparasitic
- Antitumor
- Antiviral
- Hepatoprotective
- Immunomodulating
The research on medicinal mushrooms is based on animal or in-vitro trails that are up to date. Some earlier clinical trials suggested that individuals who consume mushrooms can have the benefits of reducing cancer and it�s many symptoms in the body. There are several mechanisms that have been proposed to explain the beneficial effects of mushrooms for immune health. Certain mushrooms can positively influence the gut microbiota by protecting it from harmful pathogens. There are even several mushrooms that have been shown to support immune health by enhancing the innate and adaptive responses in the body and exerting anti-allergic effects. Here are eight mushrooms that have immune supportive properties.
The Eight Mushrooms
Chaga
The Chaga mushroom is also referred to as birch mushroom or Chaga conk. It is a dark brown and black fungus that grows on birch trees. Several beneficial compounds are found in this mushroom and contains anti-oxidant polyphenols, betulin, and betulinic acid that are associated with anti-cancer effects for the body.
Studies show that Chaga mushrooms are used in traditional medicine and can be used in different remedies. This includes using Chaga as an anthelminthic, curing digestive disorders, and to help prevent chronic illness that affects the heart and liver.
Cordyceps
Even though it is not technically a mushroom, this rare caterpillar fungus grows only in high-altitude regions in northeast India. Studies found that the bioactive components in cordyceps include polysaccharides, cordycepin, and cordycepin acid. Cordyceps was described in old Chinese medical books that traditional healers used on patients to improve their energy, stamina, and their sleeping patterns.
In a study, healthy Koreans individuals took supplements that contain cordyceps extract for eight weeks, and the results were that the extract increased the activity of NK-cells (natural killer) immune cells and improving the immune system in the body.
Lion’s Mane
Also known as Hericium Erinaceus, this mushroom has a white, fur-like appearance that resembles a lion�s mane. This mushroom can be beneficial for a healthy gut microbe and is associated with reducing colon tissue damage from inflammatory bowel disease.
Researchers suggested that lion�s mane may help individuals regulate their immune system and can improve the health of those who have IBD, but there is still more research being done to confirm this finding for the future.
Maitake
Maitake is both a culinary and medicinal mushroom that has proven to have anticancer activity for a variety of cancers that can affect the body. Maitake has a component called proteoglycan, and it has been associated with the immune-simulating effects.
Studies have been shown that proteoglycan can decrease mammary tumor cell behavior in animals and more research shows that maitake can exert anti-viral activity against hepatitis B and HIV from the body.
Oyster
Oyster mushrooms are a genus-group of fungi that has serval species like Pleurotus ostreatus and Pleurotus florida. Research has found that polysaccharides that are present in P. ostreatus mushrooms can activate N.K. cells against cancer cells. While another research shows that the extract of P. florida contains several active components containing anti-inflammatory properties in animal models.
Reishi
Known as the �king of mushrooms�, reishi has been shown to prevent various diseases and can modulate inflammation that is associated with a high cholesterol diet on people.
The health effects of this mushroom may be a result of its ability to regulate the body�s microbiota composition.� The beneficial effect that is found in reishi can help increase the beneficial bacteria that are in a person�s body.
Shiitake
Shiitake mushrooms have been traditionally used to treat common ailments that a person may encounter. Studies have shown that people who consume shiitake mushroom saw that there were changes in their body as their gut immunity and the anti-inflammatory components were improving over time.
As with many mushrooms, shiitake mushrooms have anticancer effects and lentinan that is being currently used as a complementary treatment for tumors.
Turkey Tail
The turkey tail mushroom gets its name from the tan and brown rings on its surface, resembling the tail feathers of a turkey. Research has shown that in traditional medicine, healers use the turkey tail mushroom to treat fungal infections, cancer, and AIDS on patients.
A 2007 study that was conducted by the Kyoto University Graduate School of Medicine in Japan found that over 8,000 cancer patients that took turkey tail and combined it with chemotherapy have an increased chance of survival.
Conclusion
From coming back to the body, mushrooms are used to stop diseases and cancers. Using its many health advantages of supporting the entire body can be helpful for anyone who wants to incorporate them into their diet. Mushrooms are edible while some are poisonous from the wild consuming these eight mushrooms are safe for individuals. Combining these mushrooms and some products are beneficial in supporting the immune system and are designed for more excellent stability, bioavailability, and digestive comfort.
The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues or functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or disorders of the musculoskeletal system. Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900.
References:
El-Deeb, Nehal M, et al. �Modulation of NKG2D, KIR2DL and Cytokine Production by Pleurotus Ostreatus Glucan Enhances Natural Killer Cell Cytotoxicity Toward Cancer Cells.� Frontiers in Cell and Developmental Biology, Frontiers Media S.A., 13 Aug. 2019, www.ncbi.nlm.nih.gov/pmc/articles/PMC6700253/.
Feeney, Mary Jo, et al. �Mushrooms and Health Summit Proceedings.� OUP Academic, Oxford University Press, 8 May 2014, academic.oup.com/jn/article/144/7/1128S/4569770.
Ganeshpurkar, Aditya, and Gopal Rai. �Experimental Evaluation of Analgesic and Anti-Inflammatory Potential of Oyster Mushroom Pleurotus Florida.� Indian Journal of Pharmacology, Medknow Publications & Media Pvt Ltd, 2013, www.ncbi.nlm.nih.gov/pmc/articles/PMC3608298/.
G�ry, Antoine, et al. �Chaga ( Inonotus Obliquus), a Future Potential Medicinal Fungus in Oncology? A Chemical Study and a Comparison of the Cytotoxicity Against Human Lung Adenocarcinoma Cells (A549) and Human Bronchial Epithelial Cells (BEAS-2B).� Integrative Cancer Therapies, SAGE Publications, Sept. 2018, www.ncbi.nlm.nih.gov/pmc/articles/PMC6142110/.
He, Yanli, et al. �Grifola Frondosa Polysaccharide: A Review of Antitumor and Other Biological Activity Studies in China.� Discovery Medicine, 23 Apr. 2018, www.discoverymedicine.com/Yanli-He/2018/04/grifola-frondosa-polysaccharide-antitumor-and-other-biological-activity-studies-in-china/.
Integrative, PDQ, and Alternative and Complementary Therapies Editorial Board. �Medicinal Mushrooms (PDQ�).� PDQ Cancer Information Summaries [Internet]., U.S. National Library of Medicine, 30 Nov. 2016, www.ncbi.nlm.nih.gov/books/NBK401261/.
Jayachandran, Muthukumaran, et al. �A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota.� International Journal of Molecular Sciences, MDPI, 8 Sept. 2017, www.ncbi.nlm.nih.gov/pmc/articles/PMC5618583/.
Jung, Su-Jin, et al. �Immunomodulatory Effects of a Mycelium Extract of Cordyceps (Paecilomyces Hepiali; CBG-CS-2): a Randomized and Double-Blind Clinical Trial.� BMC Complementary and Alternative Medicine, BioMed Central, 29 Mar. 2019, www.ncbi.nlm.nih.gov/pmc/articles/PMC6441223/.
Lindequist, Ulrike, et al. �Medicinal Mushrooms.� Evidence-Based Complementary and Alternative Medicine: ECAM, Hindawi Publishing Corporation, 2014, www.ncbi.nlm.nih.gov/pmc/articles/PMC4095656/.
Lindequist, Ulrike, et al. �The Pharmacological Potential of Mushrooms.� Evidence-Based Complementary and Alternative Medicine: ECAM, Oxford University Press, Sept. 2005, www.ncbi.nlm.nih.gov/pmc/articles/PMC1193547/.
Oba, Koji, et al. �Efficacy of Adjuvant Immunochemotherapy with Polysaccharide K for Patients with Curative Resections of Gastric Cancer.� Cancer Immunology, Immunotherapy: CII, Centre for Reviews and Dissemination (U.K.), June 2007, www.ncbi.nlm.nih.gov/pubmed/17106715.
Panda, Ashok Kumar, and Kailash Chandra Swain. �Traditional Uses and Medicinal Potential of Cordyceps Sinensis of Sikkim.� Journal of Ayurveda and Integrative Medicine, Medknow Publications Pvt Ltd, Jan. 2011, www.ncbi.nlm.nih.gov/pmc/articles/PMC3121254/.
Valverde, Mar�a Elena, et al. �Edible Mushrooms: Improving Human Health and Promoting Quality Life.� International Journal of Microbiology, Hindawi Publishing Corporation, 2015, www.ncbi.nlm.nih.gov/pmc/articles/PMC4320875/.
Wasser, Solomon P. �Medicinal Mushroom Science: Current Perspectives, Advances, Evidences, and Challenges.� Biomedical Journal, U.S. National Library of Medicine, 2014, www.ncbi.nlm.nih.gov/pubmed/25179726.
Do you feel:
- Aches, pains, and swelling throughout the body?
- Stomach pains, burning, or aching 1-4 hours after eating?
- Excessive belching, burping, or bloating?
- Inflammation in your stomach?
- Is gas immediately following a meal?
If you are experiencing any of these situations, then try these eight edible mushrooms for your immune system.
Mushrooms
Medicinal mushrooms have been traditionally used centuries for protecting anyone against infectious diseases, and various cancers. The positive biological effects of mushrooms are due in part to the indirect action of stimulating the immune cells. These mushrooms have a long history of usages by supporting health, especially in early Chinese, Egyptian, Greek, Mexican, and Roman cultures. In fact, in 1991, a 5,300-year-old mummy was discovered carrying polypore fungus, which exerts a purgative effect.� It may have been used to treat the mummies’ intestinal parasites.
Mushroom Benefits
Modern research has shown that medicinal mushrooms can provide a rich source of nutrients and bioactive compounds that are associated with a few health effects, primarily supporting the immune system. Mushrooms act as an anti-bacterial, immune system enhancer and cholesterol-lowering agents. Additionally, they are an essential source of bioactive compounds, and some mushroom extracts are used to promote human health as well as being found as dietary supplements.

Medicinal mushrooms are edible macroscopic fungi that are visible to the naked eye and are used for their beneficial health properties. Fungi, which includes yeasts molds, and mushrooms, live on the dead matter that is found in soil, plants, animals, and other fungi. It is estimated that there are 14000 to 22000 known species of mushrooms worldwide, and approximately 20 to 30 mushrooms that are cultivated edible species, while approximately 15 species are wild foraged for consumption and can be part as functional foods or dietary supplements.
Mushrooms are a source of many nutrients, including fiber, protein, selenium, potassium, and vitamins, B1, B2, B12, C, D, and E. They also possess several bioactive components like alkaloids, flavonoids, terpenes, phenolic compounds, polyunsaturated fatty acids, and polysaccharides. Mushrooms have been studied for not only its immune-stimulating and prebiotic properties, but they notably contain ?- glucan, which is a polysaccharide that is commonly present in mushrooms.
Research has been examining the health effects of mushrooms and has identified approximately 130 possible therapeutic properties, including:
- Anti-bacterial
- Anti-diabetic
- Anti-fungal
- Anti-inflammatory
- Anti-oxidants
- Anti-parasitic
- Anti-tumor
- Anti-viral
- Hepatoprotective
- Immunomodulating
The research on medicinal mushrooms is based on animal or in-vitro trails that are up to date. Some earlier clinical trials suggested that individuals who consume mushrooms can be beneficial for reducing the risk of breast cancer and can help improve cancer-related symptoms like insomnia and sweating.� Several mechanisms have been proposed to explain the beneficial effects of mushrooms for immune health. Certain mushrooms can positively influence the gut microbiota by improving the protection against pathogens. There are even several mushrooms that have been shown to support immune health by enhancing the innate and adaptive immune responses as well as suppressing the immune response, thereby exerting anti-allergic effects.
The Top 8 Mushrooms
Here are the top 8 mushrooms that have immune supportive properties.
Chaga (Inonotus obliquus)

The Chaga mushroom is also referred to as birch mushroom and Chaga conk. It is a dark brown and black fungus that often grows on birch trees. Several compounds are found in Chaga, with its beneficial effects that contain anti-oxidant polyphenols, betulin, and betulinic acid that are associated with anticancer effects.
Studies show that the Chaga mushrooms are used in traditional medicine for different therapeutic indications, such as using it as an anthelminthic, as an antitubercular, to cure digestive disorders (gastritis, ulcers, etc.), or even to prevent cardiac or hepatic illnesses.
Cordyceps (Ophiocordyceps Sinensis)

Even though cordyceps is not technically a mushroom, this rare caterpillar fungus grows only in high-altitude regions of Sikkim, a state in northeast India. Studies found that the bioactive components in cordyceps include polysaccharides, cordycepin, and cordycepic acid. Cordyceps was described in old Chinese medical books in ancient times and used by traditional healers to improve energy, appetite, stamina, libido, endurance, and sleeping patterns.
In an eight week study, healthy Koreans individuals took supplements that contain cordyceps extract, and the results were that with the cordyceps extract, it increased the activity of NK-cells (natural killer immune cells). This change was accompanied by improving the immune regulation in the body.
Lion’s Mane (Hericium Erinaceus)

Also known as Hericium Erinaceus, the lion’s mane mushroom has a white, fur-like appearance and may promote beneficial gut microbiota growth and be associated with reducing colon tissue damage from inflammatory bowel disease.
Researchers suggested that lion�s mane may help individuals regulate their immune system and can improve the health of those who have IBD, but there is still more research being done to confirm this finding.
Maitake (Grifola frondosa)

Maitake is both a culinary and medicinal mushroom that has proven to have anticancer activity on breast cancer, melanoma, and hepatoma cells. Maitake has a component called proteoglycan, and it has been associated with the immune-simulating effects.
Studies have been shown that proteoglycan can decrease mammary tumor cell behavior in mice, and research shows that maitake can exert anti-viral activity against hepatitis B and HIV (human immunodeficiency virus.)
Oyster (Pleurotus)

Oyster mushrooms are a genus of fungi that has serval species like Pleurotus ostreatus and Pleurotus florida.� Research has found that polysaccharides that are present in P. ostreatus mushrooms can activate N.K. cells against lung and breast cancer cells. Another research shows that an extract of P. florida contains several active components like phenolics, flavonoids, and polysaccharides having anti-inflammatory analgesic effects in animal models.
Reishi (Ganoderma lingzhi)

Known as the �king of mushrooms� or the “mushrooms of immortality,” reishi has been shown to prevent or treat various diseases and modulate inflammation that is associated with a high cholesterol diet on people.
The health effects of this mushroom may be a result of its ability to regulate microbiota composition in the body, as the polysaccharides that are found in reishi demonstrates prebiotic effects and may increase the beneficial bacteria in a person’s body.
Shiitake (Lentinula edodes)

Shiitake mushrooms have been traditionally used to treat reasonable conditions like the common cold. Studies have shown that people who consume shiitake were associated with favorable changes in secretion patterns of various immune compounds and that the changes caused by consuming shiitake mushrooms can improve the gut immunity and anti-inflammatory response.
As with many mushrooms, shiitake mushrooms have anticancer effects and contains a glucan called lentinan that is being currently used as a complementary treatment for tumors, especially in China and Japan.
Turkey Tail (Coriolus Versicolor)

The turkey tail mushroom gets its name from the tan and brown rings on its surface, and its appearance is similar to the tail feathers of a turkey. Research has shown that in traditional medicine, the turkey tail mushroom has been used to therapeutically to treat fungal infections, cancer, and AIDS (acquired immunodeficiency syndrome.) Turkey tail mushrooms have PSK (polysaccharide-K)� and have been used as a complementary cancer treatment
A 2007 study that was conducted by the Kyoto University Graduate School of Medicine in Japan found that over 8,000 patients that took turkey tail and combined it with chemotherapy have increased the survival rate of patients following gastric cancer resection.
Conclusion
Mushrooms have been used for a long time to prevent infectious diseases and various cancers from coming into the body. With its many health benefits for immune support, it can be beneficial to provide anti-inflammatory properties. Certain mushrooms are edible while others are poisonous in the wild, so consuming these eight mushrooms are safe for people. Combining these mushrooms and some products are beneficial in supporting the immune system and are designed for more excellent stability, bioavailability, and digestive comfort.
The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues or functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or disorders of the musculoskeletal system. Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900.
References:
El-Deeb, Nehal M, et al. �Modulation of NKG2D, KIR2DL and Cytokine Production by Pleurotus Ostreatus Glucan Enhances Natural Killer Cell Cytotoxicity Toward Cancer Cells.� Frontiers in Cell and Developmental Biology, Frontiers Media S.A., 13 Aug. 2019, www.ncbi.nlm.nih.gov/pmc/articles/PMC6700253/.
Feeney, Mary Jo, et al. �Mushrooms and Health Summit Proceedings.� OUP Academic, Oxford University Press, 8 May 2014, academic.oup.com/jn/article/144/7/1128S/4569770.
Ganeshpurkar, Aditya, and Gopal Rai. �Experimental Evaluation of Analgesic and Anti-Inflammatory Potential of Oyster Mushroom Pleurotus Florida.� Indian Journal of Pharmacology, Medknow Publications & Media Pvt Ltd, 2013, www.ncbi.nlm.nih.gov/pmc/articles/PMC3608298/.
G�ry, Antoine, et al. �Chaga ( Inonotus Obliquus), a Future Potential Medicinal Fungus in Oncology? A Chemical Study and a Comparison of the Cytotoxicity Against Human Lung Adenocarcinoma Cells (A549) and Human Bronchial Epithelial Cells (BEAS-2B).� Integrative Cancer Therapies, SAGE Publications, Sept. 2018, www.ncbi.nlm.nih.gov/pmc/articles/PMC6142110/.
He, Yanli, et al. �Grifola Frondosa Polysaccharide: A Review of Antitumor and Other Biological Activity Studies in China.� Discovery Medicine, 23 Apr. 2018, www.discoverymedicine.com/Yanli-He/2018/04/grifola-frondosa-polysaccharide-antitumor-and-other-biological-activity-studies-in-china/.
Integrative, PDQ, and Alternative and Complementary Therapies Editorial Board. �Medicinal Mushrooms (PDQ�).� PDQ Cancer Information Summaries [Internet]., U.S. National Library of Medicine, 30 Nov. 2016, www.ncbi.nlm.nih.gov/books/NBK401261/.
Jayachandran, Muthukumaran, et al. �A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota.� International Journal of Molecular Sciences, MDPI, 8 Sept. 2017, www.ncbi.nlm.nih.gov/pmc/articles/PMC5618583/.
Jung, Su-Jin, et al. �Immunomodulatory Effects of a Mycelium Extract of Cordyceps (Paecilomyces Hepiali; CBG-CS-2): a Randomized and Double-Blind Clinical Trial.� BMC Complementary and Alternative Medicine, BioMed Central, 29 Mar. 2019, www.ncbi.nlm.nih.gov/pmc/articles/PMC6441223/.
Lindequist, Ulrike, et al. �Medicinal Mushrooms.� Evidence-Based Complementary and Alternative Medicine: ECAM, Hindawi Publishing Corporation, 2014, www.ncbi.nlm.nih.gov/pmc/articles/PMC4095656/.
Lindequist, Ulrike, et al. �The Pharmacological Potential of Mushrooms.� Evidence-Based Complementary and Alternative Medicine: ECAM, Oxford University Press, Sept. 2005, www.ncbi.nlm.nih.gov/pmc/articles/PMC1193547/.
Oba, Koji, et al. �Efficacy of Adjuvant Immunochemotherapy with Polysaccharide K for Patients with Curative Resections of Gastric Cancer.� Cancer Immunology, Immunotherapy: CII, Centre for Reviews and Dissemination (U.K.), June 2007, www.ncbi.nlm.nih.gov/pubmed/17106715.
Panda, Ashok Kumar, and Kailash Chandra Swain. �Traditional Uses and Medicinal Potential of Cordyceps Sinensis of Sikkim.� Journal of Ayurveda and Integrative Medicine, Medknow Publications Pvt Ltd, Jan. 2011, www.ncbi.nlm.nih.gov/pmc/articles/PMC3121254/.
Valverde, Mar�a Elena, et al. �Edible Mushrooms: Improving Human Health and Promoting Quality Life.� International Journal of Microbiology, Hindawi Publishing Corporation, 2015, www.ncbi.nlm.nih.gov/pmc/articles/PMC4320875/.
Wasser, Solomon P. �Medicinal Mushroom Science: Current Perspectives, Advances, Evidences, and Challenges.� Biomedical Journal, U.S. National Library of Medicine, 2014, www.ncbi.nlm.nih.gov/pubmed/25179726.
Zaremba, Karolina. �Top 8 Mushrooms For Immune Health.� Fullscript, 4 Nov. 2019, fullscript.com/blog/mushrooms-for-immune-health.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Health, Mental Health, Mind Body and Spirit
Do you feel:
- That your joints are aching for no reason?
- Depression/lack of motivation?
- Inflammation in your joints?
- Feel cold?
- Edema?
If you are experiencing any of these situations, then it might be the weather that is affecting your mood and your body.
The Weather
Does the weather forecast make anyone smile? Whether it is nothing but bright, sunny skies and warm temperatures or gray, overcast skies with threats of rain and thunderstorms, the weather can affect a person’s joints and cause them pain. The old saying “Feel it in my bones” comes to play when environmental conditions can affect the physical body. Research has indicated that these effects are not just skin deep, but the weather can affect a person�s mood and emotional health. They found that patients experience increased joint pain in response to a decrease in pressure and indicating that low atmospheric pressure conditions exacerbate joint pain.

Lots of people are affected differently by different weather patterns. There are no hard-fast rules regarding the influence of how the weather affects people�s moods. The research suggested that high humidity may increase sleepiness and can negatively affect concentration and focus on a person. While rising temperatures can help lower anxiety and skepticism mood scores in a person. Since humidity is the most significant predictor since it implicates for school and office performances are being discussed and highlights the importance of humidity as a weather variable.

Some individuals love to sit out in the sun and soak up every ray while basking in the heat. Others instead prefer to let themselves stay indoors surrounded by air conditioning and feeling so much better in the colder weather with less sunshine.
Types of People Affected By The Weather
Studies have researched that there were four distinct types of people, especially in children and their mothers that were identified when it comes to the weather and their moods. They are:
- Summer Lover
- Unaffected
- Summer Haters
- Rain Haters
Summer Lovers have better moods in warm, sunny weather while the Summer Hater has the worse moods under the warmer conditions. People in the Unaffected category has shown only the weak association between the weather and their moods. When it comes to rainy days, Rain Haters experiences particularly bad moods during those types of days. The correlation between the children and their mothers was founded for two of the types. It stated that there might be some intergenerational influences, and the finding from the study and many others show that there is a massive individual difference in how the weather affects people’s moods. Some people love rainy or sunny days, while others loathe them.

A 2013 paper found that rising temperatures and increased precipitation can have a significant impact on human conflict and interpersonal violence. The correlation between the higher temperatures like more extreme rainfall and increased violence was seen on both scales, large and small. Other researchers have suggested that the psychological effects of the weather are influenced by seasons and the time a person is outside. What they found was that higher temperatures or the barometric pressure were related to better moods, memory, and “broadened” cognitive style in the springtime as an individual spends more time outside has increased.
Weather Can Affect People�s Mood
While this relationship is perfect for some people, others see this relationship as an inverse during the other seasons. Some people found out that during the warmer seasons, lowers their mood. It is correlated strongly with individuals who live in the south. The hotter weather can cause them to have poorer moods when the summer has higher temperatures, and it can become downright debilitating.

Researchers speculated that the discrepancy between spring and summer moods might be related to seasonal affective disorder. With seasonal affective disorder, the results were consistent with their findings. They suggested that pleasant weather improves moods and broaden cognition in the spring because people have been deprived of such weather during the winter.
The founder and editor-in-chief of Psych Central, John M, Grohol, Psy.D., noted that the weather could affect people’s moods and emotions. He also mentions that the strength of that relationship varies from person to person, and the effects are noticeable, whether it be small in some people or more pronounced in others.

Another study found that many people intuit that the bad weather makes them sad and pleasant weather makes them happy. Scientific investigations have largely failed to support such associations, however, with variations in meteorological variables either showing no or weak relationships with variations in normal moods. It means that a person�s definition of� �good� or �bad� weather is their own opinion. If someone likes the rain, then gray, rainy days are �good� in their view while others view rainy days are �bad� and prefer sunshine, blue skies, and warmer weather.
Conclusion
The weather can affect anyone’s mood. Whether people enjoy the colder seasons or the warmer seasons, their moods can change due to the type of weather. If they are aware of their mood patterns, taking supplements can ease the transition of the change of seasons and be a beneficial impact on their moods. Some products can help support the body and making sure that the entire system is functioning correctly by targeting amino acids and sugar metabolism.
The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues or functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or disorders of the musculoskeletal system. Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900.
References:
Bullock, Ben, et al. �Highs and Lows, Ups and Downs: Meteorology and Mood in Bipolar Disorder.� PloS One, Public Library of Science, 9 Mar. 2017, www.ncbi.nlm.nih.gov/pmc/articles/PMC5344507/.
Grohol, John M. �Weather Can Change Your Mood.� World of Psychology, 28 Mar. 2019, psychcentral.com/blog/weather-can-change-your-mood/.
Howarth, E, and M S Hoffman. �A Multidimensional Approach to the Relationship between Mood and Weather.� British Journal of Psychology (London, England: 1953), U.S. National Library of Medicine, Feb. 1984, www.ncbi.nlm.nih.gov/pubmed/6704634.
Hsiang, Solomon M., et al. �Quantifying the Influence of Climate on Human Conflict.� Science, American Association for the Advancement of Science, 13 Sept. 2013, science.sciencemag.org/content/341/6151/1235367.
Keller, Matthew C, et al. �A Warm Heart and a Clear Head. The Contingent Effects of Weather on Mood and Cognition.� Psychological Science, U.S. National Library of Medicine, Sept. 2005, www.ncbi.nlm.nih.gov/pubmed/16137259.
Keller, Matthew C, et al. �A Warm Heart and a Clear Head. The Contingent Effects of Weather on Mood and Cognition.� Psychological Science, U.S. National Library of Medicine, Sept. 2005, www.ncbi.nlm.nih.gov/pubmed/16137259.
Klimstra, Theo A, et al. �Come Rain or Come Shine: Individual Differences in How Weather Affects Mood.� Emotion (Washington, D.C.), U.S. National Library of Medicine, Dec. 2011, www.ncbi.nlm.nih.gov/pubmed/21842988.
Team, DFH. �Weather Forecast � Can It Predict Your Mood, Too?� Designs for Health, 15 Aug. 2019, blog.designsforhealth.com/node/1085.
Verg�s, Josep, et al. �Weather Conditions Can Influence Rheumatic Diseases.� Proceedings of the Western Pharmacology Society, U.S. National Library of Medicine, 2004, www.ncbi.nlm.nih.gov/pubmed/15633634.
by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gastro Intestinal Health, Gut and Intestinal Health, Health, Wellness
Do you feel:
- That eating relieves fatigue?
- Hormone imbalances?
- Aches, pains, and swelling throughout the body?
- Bodily swelling for no reason?
- Inflammation on your body?
If you are experiencing any of these situations, you might be experiencing inflammation, and it might affect your endocrine system.
Inflammation and the Endocrine System
Inflammation is a defense mechanism in the body. The immune system can recognize the damaged cells, irritants, and pathogens that cause harm to the body and began the healing process. When the inflammation turns into chronic inflammation, it can cause several diseases and conditions in the body and can cause harm to an individual.
Inflammation can cause dysfunction when it is in the endocrine system. The endocrine system is a series of glands that produce and secretes hormones that the body needs and uses for a wide range of functions. When the endocrine glands produce hormones, they are sent into the bloodstream to the various tissues in the body. Once they are in the various tissues, the hormone signals the tissues to tell them what they are supposed to do. When the glands do not produce the right amount of hormones, various diseases like inflammation can affect the body.
Inflammation Symptoms and Causes
Two questions are asked concerning the interaction of the endocrine system with inflammation: How does inflammation influences the endocrine system, and does it influences disease? How do hormones influence inflammation and immune cells? A theory had integrated both questions and has recently been demonstrated in the context of chronic inflammation considering a rheumatic disease.
So how does inflammation influence the endocrine system? Inflammation symptoms can vary depending on if it is acute or chronic. The effects of acute inflammation are summed up by the acronym PRISH. They include:
- Pain: The inflamed area is most likely to be painful, especially during and after touching. The chemicals that stimulate the nerve endings are released, making the area more sensitive.
- Redness: This occurs due to the capillaries in the area that is filled with more blood than usual.
- Immobility: There may be some loss of function in the region of the inflammation where the injury has occurred.
- Swelling: A buildup of fluid causes this.
- Heat: Heat is caused by having more blood flow to the affected area and making it warm to the touch.
These acute inflammation signs only apply to an inflammation on the skin. If the inflammation occurs deep inside the body, like the endocrine system and the internal organs, some of the signs may be noticeable. Some internal organs may not have sensory nerve endings nearby; for example, they will not have pain.
With the effects of chronic inflammation, it is long term and can last for several months or even years. The results from chronic inflammation can be from:
- An autoimmune disorder that attacks normal healthy tissue and mistaking it for a pathogen that causes diseases like fibromyalgia.
- An industrial chemical that is exposed to a low level of a particular irritant over a long period.
- Failure to eliminate whatever was causing acute inflammation.
Some of the symptoms of chronic inflammation can be present in different ways. These can include:
- Fatigue
- Mouth sores
- Chest pains
- Abdominal pain
- Fever
- Rash
- Joint pain
When inflammation affects the endocrine system, it can cause the body’s system to be unbalanced, and it can lead to chronic long term illnesses.
With the second question, it is asking how do hormones influence inflammation and the immune system? When the hormone levels are either too high or too low, it can have several effects on a person’s health. The signs and symptoms can depend on hormones that are out of balance.
Inflammation and Hormones
Research has shown that some of the conditions that are affecting the endocrine system can lead to autoimmune disorders. High levels of hormones can lead to hyperthyroidism, Cushing syndrome, and Graves disease. While low levels of hormones can lead to hypothyroidism and Addison disease. When the levels of the hormones are either too high or too low, the body fluctuates from either weight gain or weight loss and disrupting the glucose levels. This can cause a person to get diabetes and obesity.
Obesity is the main risk factor for type 2 diabetes. During the development of obesity, subclinical inflammatory activity in the tissues are activated and involves the metabolism and energy homeostasis. In the body, intracellular serine/threonine kinases are activated in response to those inflammatory factors. They can catalyze the inhibitory phosphorylation of the key proteins of the insulin-signaling pathway, leading to insulin resistance in the body.
Studies have shown that inflammation is a general tissue response to a wide variety of stimuli. When inflammation is not adequately regulated, inflammatory responses may be exaggerated or ineffective, which can lead to immune dysfunction, recurring infections, and tissue damage, both locally and systemically. With various hormones, cytokines, vitamins, metabolites, and neurotransmitters being key meditators of the immune and inflammatory responses to the endocrine system.
Another study shows that aging, chronic psychological stress, and mental illnesses are also accompanied by chronic smoldering inflammation. Chronic smoldering inflammation in humans is already established with elevations of serum levels, leading to an increase in resting metabolic rate.
Conclusion
So inflammation is a double edge sword where it can heal the body but also cause the body harm if it is deep into the internal organs and body systems. With the endocrine system, the levels of the hormones can fluctuate from going too high or too low and affecting the tissues in the body, causing inflammation. �When an individual is suffering from chronic inflammation, it can change their lifestyle drastically. Some products are here to help counter the metabolic effects of temporary stress and make sure that the endocrine system is supported as well.
The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues or functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or disorders of the musculoskeletal system. Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900.
References:
Coope, Andressa, et al. �MECHANISMS IN ENDOCRINOLOGY: Metabolic and Inflammatory Pathways on the Pathogenesis of Type 2 Diabetes.� European Journal of Endocrinology, U.S. National Library of Medicine, May 2016, www.ncbi.nlm.nih.gov/pubmed/26646937.
Felman, Adam. �Inflammation: Causes, Symptoms, and Treatment.� Medical News Today, MediLexicon International, 24 Nov. 2017, www.medicalnewstoday.com/articles/248423.php.
Salazar, Luis A., et al. �The Role of Endocrine System in the Inflammatory Process.� Mediators of Inflammation, Hindawi, 29 Sept. 2016, www.hindawi.com/journals/mi/2016/6081752/.
Seladi-Schulman, Jill. �Endocrine System Overview.� Healthline, 22 Apr. 2019, www.healthline.com/health/the-endocrine-system.
Straub, Rainer H. �Interaction of the Endocrine System with Inflammation: a Function of Energy and Volume Regulation.� Arthritis Research & Therapy, BioMed Central, 13 Feb. 2014, www.ncbi.nlm.nih.gov/pmc/articles/PMC3978663/.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Chiropractic, Fibromyalgia, Functional Medicine, Wellness
Do you feel:
- Afternoon fatigue?
- Headaches with exertion or stress?
- Can you not stay asleep?
- Slow starter in the morning?
- Afternoon headaches?
If you are experiencing any of these situations, then you might be experiencing fibromyalgia.
Fibromyalgia is a common and chronic syndrome that causes pain and mental distress in the body. It causes widespread musculoskeletal pain, and it is accompanied by fatigue, sleep memory, and mood issues to the body.�The symptoms may be similar to arthritis; however, fibromyalgia is a rheumatic condition and causes soft tissue pain or myofascial pain.
The NIAMS (National Institute of Arthritis and Musculoskeletal and Skin Diseases) stated that around 5 million adults, ages 18 years and over in the United States had experienced fibromyalgia. Around 80 to 90 percent of fibromyalgia patients are mostly found in women who have this chronic disease, and men can have it as well.
Symptoms of Fibromyalgia
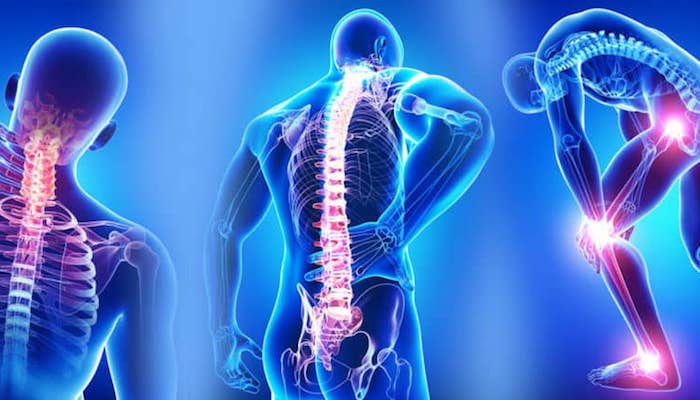
There are three symptoms that fibromyalgia causes a person to have discomfort in their daily lives. They are:
- Widespread pain: This pain is associated with fibromyalgia, and it is described as a constant dull ache that can last for at least three months. For it to be considered as widespread pain, it must occur both sides of the body, as well as above and below the waist.
- Fatigue: Individuals with fibromyalgia often awaken tired, even though they have been sleeping for long periods. The pain that fibromyalgia causes can disrupt the person’s sleep patterns, causing them to have sleep disorders such as restless legs syndrome and sleep apnea.
- Cognitive difficulties: This symptom is commonly known as �fibro fog.� It impairs the person�s ability to focus, pay attention, and concentrate on mental tasks.
Other symptoms can include:
- IBS (irritable bowel syndrome)
- Stiff joints and muscles in the morning
- Headaches
- Problems with vision
- Nausea
- Pelvic and urinary problems
- Depression and anxiety
In the past, studies have shown that patients diagnosed with fibromyalgia had 11 out of 18 specific trigger points all around their bodies. Healthcare providers would check their patients and document how many of these points were painful to their patients by firmly, but gently, pressing their bodies to get a diagnosis.
The typical trigger points include:
- The back of the head
- The tops of the shoulders
- The upper chest
- The hips
- The knees
- The outer elbows
Nowadays, in a 2016 revised diagnostic criteria, healthcare providers can diagnose patients with fibromyalgia if the patient has pain in 4 out of 5 areas of the body that is causing them pain. The protocol when diagnosing patients is referred to as �multisite pain.�
Fibromyalgia Affecting the Endocrine System
When it comes to fibromyalgia, the symptoms are associated with the endocrine system. Research shows that fibromyalgia-like symptoms such as muscle pain and tenderness, exhaustion, reduced exercise capacity, and cold intolerance can resemble symptoms that are associated with the endocrine dysfunction like hypothyroidism and adrenal or growth hormone insufficiency.
More research has stated that fibromyalgia causes a person to develop chronic fatigue syndrome. Chronic fatigue syndrome causes the body to have a deficiency of serotonergic activity and the hypofunction of sympathetic nervous system function that could contribute to the abnormalities of the central components of the HPA axis. It can distort the body’s hormonal pattern that is being attributed to the hyperactivity of the CRH neurons. The hyperactivity caused by the CRH neurons can be driven and sustained by stress being exerted by chronic pain that has originated in the musculoskeletal system or the alternation of the central nervous system mechanism of nociception.

Researchers believe that repeated nerve stimulation causes the brains of patients with fibromyalgia to change. The change causes an abnormal increase level of certain chemicals (neurotransmitters) in the brain that signals pain. In addition, the brain�s pain receptors will develop a sort of memory of the pain that is causing problems to the patient�s body and causing them to be more sensitive since the signals are overreacting.
Treating Fibromyalgia

Even though fibromyalgia pain can be uncomfortable and consistent enough to interfere with a person�s daily routine. There are ways to relieve the pain and inflammation that fibromyalgia causes the body. Pain medication can bring down the inflammation and help a person sleep a little better. Other safe treatments that can help manage fibromyalgia pain are:
- Acupuncture: Acupuncture is a Chinese medical system that uses needles to cause changes in the blood flow and the levels of neurotransmitters in the brain and spinal cord.
- Therapy: A variety of different therapies can help a person reduce the effects that fibromyalgia has caused.
- Yoga and tai chi: These practices combine meditation, slow movements, deep breathing, and relaxation � both help control fibromyalgia symptoms.
- Reducing stress: Developing a plan to avoid or limit overexertion and emotional stress is useful when dealing with fibromyalgia. Learning to meditate and trying stress management techniques can help a person feel calm and recharged for the rest of the day.
- Getting enough sleep: Since fatigue is one of the main characteristics of fibromyalgia, getting enough sleep is essential. Practicing good sleep habits and going to bed and getting up at the same time each day can lessen the effects of fatigue.
- Exercising regularly: At first, exercising may increase the pain, but doing it gradually and regularly over time can decrease the symptoms. This can be walking, swimming, biking, and water aerobics can be beneficial to the body.
- Pacing yourself: Keeping track of activities is beneficial for people with fibromyalgia. Moderation of daily activities on the good days can help a person overcome the symptoms when they flare-up.
- Maintaining a healthy lifestyle: Eating healthy food that has anti-inflammatory properties can be useful for the body, and finding enjoyable hobbies can be beneficial as well.
Conclusion
Fibromyalgia is a chronic illness that causes pain and inflammation that affects the soft tissue in the body. The symptoms can resemble joint inflammation and causes people to have fatigue and discomfort all over their body. When these symptoms flare up, it can cause body damage. Treatments can help a person reduce the effects of fibromyalgia and be beneficial. Some products are formulated to counter the effects of temporary stress and offer support in the gastrointestinal system and the body�s metabolism.
The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues as well as functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or chronic disorders of the musculoskeletal system. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at�915-850-0900�.
References:
Felman, Adam. �Fibromyalgia: Symptoms, Causes, and Treatment.� Medical News Today, MediLexicon International, 5 Jan. 2018, www.medicalnewstoday.com/articles/147083.php.
Geenen, Rinie, et al. �Evaluation and Management of Endocrine Dysfunction in Fibromyalgia.� Rheumatic Diseases Clinics of North America, U.S. National Library of Medicine, May 2002, www.ncbi.nlm.nih.gov/pubmed/12122926.
Neeck, G, and L J Crofford. �Neuroendocrine Perturbations in Fibromyalgia and Chronic Fatigue Syndrome.� Rheumatic Diseases Clinics of North America, U.S. National Library of Medicine, Nov. 2000, www.ncbi.nlm.nih.gov/pubmed/11084955.
Staff, Mayo Clinic. �Fibromyalgia.� Mayo Clinic, Mayo Foundation for Medical Education and Research, 11 Aug. 2017, www.mayoclinic.org/diseases-conditions/fibromyalgia/symptoms-causes/syc-20354780.
Staff, Mayo Clinic. �Fibromyalgia.� Mayo Clinic, Mayo Foundation for Medical Education and Research, 11 Aug. 2017, www.mayoclinic.org/diseases-conditions/fibromyalgia/diagnosis-treatment/drc-20354785.
Unknown, Unknown. �Fibromyalgia.� National Institute of Arthritis and Musculoskeletal and Skin Diseases, U.S. Department of Health and Human Services, 30 Sept. 2019, www.niams.nih.gov/health-topics/fibromyalgia.
Wolfe, Frederick, et al. �2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria.� Seminars in Arthritis and Rheumatism, W.B. Saunders, 30 Aug. 2016, www.sciencedirect.com/science/article/abs/pii/S0049017216302086?via%3Dihub.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Chiropractic, Functional Medicine, Health, Health Coaching, Wellness
Health Coaching is a recent position that is being utilized by doctor offices around the country. Many doctors have realized that their patients are needing more one on one guidance but they are unable to provide this due to their hectic schedule. This is where they have created and utilized health coaches.
Health coaching is extremely beneficial for patients and can help them achieve their health goals. For more information about health coaches and a general overview of the essential role they play in the healthcare field, please see last week’s article linked here.
Health coaches use many different techniques depending on the patient they are working with, but the core values of their methods remain the same. These core values can be broken down into 6 different steps, with each individual step having smaller more detailed steps of their own. These steps can be identified as:
Identifying values and vision
Determining goals
Building a plan for action
Tracking progress
Visualizing one’s best self
Creating a plan for resiliency
Step 1: Identify Values

With this step being the first, it is one of the most crucial. When a patient comes to a physician or a health coach, it is usually because they have been recently diagnosed or are unhappy with their current health status. However, this does not mean that the patient is fully ready to accept their condition or understands it fully.
The patient will be asked to write down inventory in the categories of physical, emotional, spiritual, social, recreational, intellectual, and environmental. The purpose of this is so the patient is able to search and reflect on where they’re currently at and where they would like to be.
From here, there are different techniques and models that a coach may use. One being the transtheoretical model, in which the patient will use stages to move through a behavior change.
At this point, the conversation is less about treatment and more about obtaining an awareness of their health risks, experience with a current illness or any symptoms they�re experiencing. The patient is welcome and encouraged to express their emotions openly. The health coach will move through these next 6 steps to help outline the patient’s treatment and see what stage they are at.
1. Precontemplation: the patient does not intend to take action in the foreseeable future
2. Contemplation: intending to start introducing healthy behaviors within the next 6 months
3. Preparation: patients are ready to take action in the next 30 days
4. Action: the patient has recently changed their behavior and intends to keep moving forward
5. Maintenance: the patient has sustained their behavior change for 6 months and intends to maintain the behavior change for more than six months
6. Termination: the patient has grown and is now self-aware of their behaviors and has no desire to return to their previously unhealthy behaviors
As we all know, values are formed starting in early childhood. These values are then later consciously re-evaluated and may change. It is important for the patient to work with the health coach so they properly understand their personal values. This allows the patient to get clarity and build self-awareness to make intelligent decisions and keep a balance in life.
Actually sitting down identifying values might be difficult, as many individuals do not think about them often. If this is the case, the health coach might help by asking questions like:
What is more important in your life: Beyond basic human needs, what must you have in your life to experience fulfillment?
Take this time and consider a meaningful moment: What was happening to you and what values were you honoring?
Consider a time when you were angry or upset: What were you feeling and, if you flip those feelings around, what value is being suppressed?
These questions aid in triggering times that the patient might not have been connecting to values. After the patient has identifies values, the health coach will work with the patient to select between 5-10 of their core values and then rank them in order of importance. From here, the patient is able to evaluate their values and proceed to determining goals.
Step 2: Determine Goals

Once a patient has identified their values, the health coach will shift their focus and have them brainstorm what they would like to focus on in their healing plan. This step is important because it will determine what they specifically want to change or improve. Some patients may feel unsure or are apprehensive, but allowing the patient time to journal or write out everything they want to accomplish, big or small, as well as the known steps or tasks, will help the patient get there.
When determining goals, the health coach will encourage the patient to create goals for multiple areas in their life. Some of these areas may include, health, family, relationships, and recreation. The health coach will encourage the patient’s goals by having them consider the following questions:
What do I want to achieve?
How will I achieve this goal?
Why do I want to reach this goal?
Who will I need to work with to achieve this goal?
What are the conditions and limitations to achieving this goal?
Based on the core areas and goals, the health coach will work with the patient to determine SMART goals. A SMART goal is a goal-setting technique that brings structure and trackability to goals. SMART goals stand for Specific, Measurable, Attainable, Relevant, and Timely. These create a verifiable trajectory towards a specific objective with clear milestones. By determining SMART goals, it clarifies how and when the goal will be achieved, rather than just stating a desire.
A health coach will help patients turn “I want to lose weight” into ” I want to lose 20 pounds to have more energy to play with my grandchildren. I will do so by exercising four times a week and eating less processed foods, and more fruits and vegetables. I will lose an average of two pounds every week for 10 weeks.”
By doing this, the health coach is working on a goal that immediately interests the patient and puts it into a way that is more attainable. The health coach can help the patient stay encouraged and motivated as they work together to achieve small successes, eventually leading to the patient being more willing to take on bigger challenges.
Using health coaching can be more beneficial than one might originally think. As one can see, they really go deep into one’s life and can help them in ways they might not have planned on originally. In the next article, the steps of building a plan for action and tracking progress and results will be discussed in great detail.
Changing your lifestyle can be difficult and does not happen overnight. Those who work with a coach to reach their goals are more successful and less likely to give up when things get difficult. Coaches are amazing for accountability, advice, health help, goal setting, and organizing expectations in a realistic timeline. Look at it this way: People use a wedding coordinator to help them oragnize food, timelines, expectations, etc. and that is for an event that lasts 1 day. So why are you not using a health coach to help you organize all of these same things for something that will last a lifetime? In addition you’re getting to help decide your future and gain a deeper undestanding of what lies ahead. Investing in yourself is one of the best things you can do for yourself. – Kenna Vaughn, Senior Health Coach
The scope of our information is limited to chiropractic, musculoskeletal and nervous health issues as well as functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or chronic disorders of the musculoskeletal system. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900.
The information from this article was found in an article written by Integrative Practioner. The sources can be found listed below.
Resources:
American Psychological Association (2019). The Road to Resilience. Retrieved from: https://www.apa.org/helpcenter/road-resilience
Jonas, W. (2019). Empowering patients with chronic diseases to live healthier through health coaching: Integrative primary care case study. Samueli Integrative Health Programs.Retrieved from: https://www.health.harvard.edu/staying-healthy/give-yourself-a-health-self-assessment
Miller, W. and Rose, G. (1991). Motivational Interviewing: Preparing People to Change Addictive Behavior. Guilford Publications.
Pecoraro, Wendy. �A Six-Step Approach To Health And Wellness Coaching: A Toolkit for Practice Implementation.� Integrative Practitioner.Com, 2019.
Trzeciak, S. and Mazzarelli, A. (2019). Compassionomics. Studer Group. Virginia Polytechnic Institute and State University. The Stages of Change.�Retrieved from: http://www.cpe.vt.edu/gttc/presentations/8eStagesofChange.pdf
Your Coach (2009). SMART goals. Retrieved from: https://www.yourcoach.be/en/coaching-tools/









































