
by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gastro Intestinal Health, Gut and Intestinal Health, Health
For anyone that has dealt with mold knows that it is mostly found in fresh produce when it hasn’t been eaten. It is even there is a new damp spot in the house, and it�s left untreated. Mold is a type of fungus that is presented everywhere, including the air. It can actually cause someone highly sensitive to mold exposure to have chronic raspatory illnesses like asthma and bronchitis.
Studies show that the most common species of mold is Stachybotrys chartarum or black mold. This type of fungus thrives in warm, moist environments, including the basement, the bathroom, and the kitchen. It releases toxins in the air that is irritating or harmful to individuals with existing health conditions and becoming mycotoxin.
What is Mycotoxin?

A mycotoxin is a secondary metabolite being produced by organisms of the fungal kingdom. It can move in and out of cells in the body, causing inflammation when it is indigested. Researchers suggest that mycotoxin can link to serious health problems to people who live in contaminated buildings, and it can have long-term results. In most cases, mycotoxin can cause problems in the gut by consuming moldy food; causing leaky gut and destroying the gut microflora.
Here are some of the symptoms of mycotoxin:
- Aches and pains
- Mood changes
- Headaches
- Brain fog
- Asthma
- Watery, red eyes
- Runny or blocked nose
- Gut inflammation
- Sore throat
They are teratogenic, mutagenic, nephrotoxic, immunosuppressive, and carcinogenic. They can cause DNA damage, cancer, immune suppression, neurological issues, and a variety of adverse health effects on the human body. With mycotoxins, they have spores and pieces of hyphae that releases toxins into the air. They are tiny, but they are not easily detectable in the bloodstream since they can attach themselves to enzymes that are involved in insulin receptors. This results in dysfunction the in cells ability to intake and process glucose in the gut.
When mycotoxin is in the gut, it damages the intestinal barrier. It can cause malabsorption of food and disrupts the protein synthesis. When that happens, the individual�s autoimmunity will rise up, causing their bodies to go into overdrive to fight the problem.
Mycotoxin can actually grow in grains such as rice. The fungal mycotoxin has been known to cause liver damage since the contaminated food is being consumed by people, and it creates a rise in inflammation. When this happens, individuals start being sensitive to the contaminated foods that they are consuming. There is still more research to mycotoxin that is being produced to create a resistance to mycotoxin exposure.
Diagnosing Mycotoxin
Mycotoxin can�t be diagnosed by the symptoms themselves, doctors can perform one of these tests to determine the severity of mycotoxins in individuals.
- Blood test: Physicians can take a patient�s blood sample and send it to a testing lab to test. This is to see if there is a reaction of specific antibodies in the patient�s immune system. A blood test can even check the individual�s biotoxins in their blood to see if mycotoxin present.
- Skin prick test: Healthcare professionals can take tiny amounts of mold and use a small needle to apply it onto the patient�s skin. This is to determine if the individual is breaking out in bumps, a rash or hives, then they are allergic to any mold species.
Diagnosing mycotoxin is known by many names, but it is mostly called mast cell disorder. Even though they are different and have different manifestations, diagnosing them in the body is essential to help individuals to heal their ailments. With technology getting better, healthcare physicians can detect mycotoxins in the body much faster.
Treating Mycotoxin
There are many ways to treat mycotoxin. Options include:
- Avoiding the mold whenever possible.
- A nasal rinse to flush out the mold spores that are in the nose.
- Antihistamines to stop the itchiness, runny noses, and sneezing due to mold exposure.
- A short term remedy for congestion is using decongestant nasal spray.
- Montelukast is an oral medication to reduce the mucus in a patent�s airways to lower the symptoms for both mold allergies and asthma.
- Doctors can recommend patients an allergy shot to build up the patient�s immunity to mycotoxin if the exposure is long term.
How to check for mycotoxin?
When individuals are checking for mycotoxins in their environment, it is best to hire professionals to help identify and remove it. A lot of individuals can look for black clusters growing in warm, moist rooms and can search for the causes of mold growth like any leaks, old food, papers, or wood. People can throw away the items that are affected by mold or that are contributing to mold growth. They can also remove the things that are not affected by mold exposure.

Wearing a mold-resistant suit, mask, gloves, and boots can protect individuals as they are getting rid of mildew and mold from their environment. Even purchasing a HEPA air purifier can help get rid of the spores to ensure that no allergens will affect the body�s immune system. When individuals are removing the mold exposure out of the affected area, they can cover the non-affected surfaces with bleach or a fungicidal agent. Then let it dry to prevent the mold from reproducing on the same area it has infected.
Conclusion
Since researchers are still doing a test on mycotoxin, mold exposure is still all around the world and in many forms. It can even contaminate food and places where it can thrive and grow. Individuals can prevent it from locating the source and can take precautions when they are exposed to the spores. If the individual is exposed to mycotoxin, going to the doctors to get tested is the best route to go. The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues as well as functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or chronic disorders of the musculoskeletal system. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900 .
References
Borchers, Andrea T, et al. �Mold and Human Health: a Reality Check.� Clinical Reviews in Allergy & Immunology, U.S. National Library of Medicine, June 2017, www.ncbi.nlm.nih.gov/pubmed/28299723.
Do�en, Ina, et al. �Stachybotrys Mycotoxins: from Culture Extracts to Dust Samples.� Analytical and Bioanalytical Chemistry, Springer Berlin Heidelberg, Aug. 2016, www.ncbi.nlm.nih.gov/pmc/articles/PMC4939167/.
Gautier, C, et al. �Non-Allergenic Impact of Indoor Mold Exposure.� Revue Des Maladies Respiratoires, U.S. National Library of Medicine, June 2018, www.ncbi.nlm.nih.gov/pubmed/29983225.
Hurra�, Julia, et al. �Medical Diagnostics for Indoor Mold Exposure.� International Journal of Hygiene and Environmental Health, U.S. National Library of Medicine, Apr. 2017, www.ncbi.nlm.nih.gov/pubmed/27986496.
Jewell, Tim. �Black Mold Spores and More.� Black Mold Exposure, 1 June, 2018, www.healthline.com/health/black-mold-exposure.
Leonard, Jayne. �Black Mold Exposure: Symptoms, Treatment, and Prevention.� Medical News Today, MediLexicon International, 17 Sept. 2019, www.medicalnewstoday.com/articles/323419.php.
Pitt, John I, and J David Miller. �A Concise History of Mycotoxin Research.� Journal of Agricultural and Food Chemistry, U.S. National Library of Medicine, 23 Aug. 2017, www.ncbi.nlm.nih.gov/pubmed/27960261.
Sun, Xiang Dong, et al. �Mycotoxin Contamination of Rice in China.� Journal of Food Science, U.S. National Library of Medicine, Mar. 2017, www.ncbi.nlm.nih.gov/pubmed/28135406.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Chiropractic, Functional Medicine, Health
The Neural Zoomer Plus is a blood test that is designed to test 48 neurological antigens. When testing for these antigens, the results these markers find can help physicians determine if a patient is at risk for neurological conditions later on. To view last week’s article containing a full list of the signs and 48 markers, click here.�
When a patient comes to us with concerns, we listen very intently and make sure our patient’s concerns are addressed. More often than not, patients reveal they are having issues that relate to neurological declines, such as, muscle spasms or memory loss. With symptoms like these, the patient is referred to get a Neural Zoomer Plus.�
Once we receive the results back, it is compiled into a large report. From here, we assess it and go through all of the markers with an additional team of clinicians. An example of a few of the markers tested in a Neural Zoomer Plus is below. One can see that this patient has an elevated �Anti-Voltage gated potassium channel�. Anti- Voltage-gated potassium channels are responsible for multiple cellular processes such as cell growth and differentiation.�
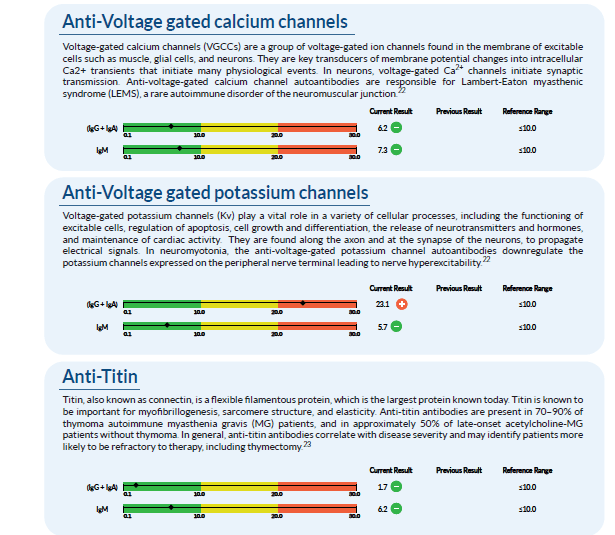
After analyzing the data, we take these findings and create a treatment protocol that is specific to each patient and their lifestyle. Due to the fact that this patient shows an elevation in specific markers, there are certain adjustments we make in order to help prevent or reverse the cognitive and physical effects of neurological disorders.�
The first step we take is to change the diet of the patient. Many foods are not properly digested, leading to gut inflammation, which further leads to �leaky gut� which then enters the bloodstream and into the blood-brain barrier, causing neurological decline. In order to reverse these effects, we want to make sure the gut is getting the proper nutrients from foods that will not cause inflammation. For this patient, we recommend the Wahls Protocol.�
The second step we take is to usually get the patient involved in a more active lifestyle. By having this patient start to exercise with activities like yoga, it can improve the state of mind and their mind-body connection.�
The third step is usually nutraceuticals. These are supplements that will naturally help the body and brain with no addictive or harsh chemicals. With every patient, the nutraceuticals and amount needed vary depending on their specific body. For this case, we recommend:��
N-acetyl-L-cysteine:� (NAC) is a precursor to glutathione, the body�s most important cellular�antioxidant. NAC supplements have been shown to increase cellular glutathione levels.
Vitamins B12, B6, and folate: These are metabolic cofactors important for cellular metabolism and maintenance of all tissue cell types, but particularly important to nerve cells. Deficiencies in�B12 or folate can raise homocysteine levels, which have been associated with a higher risk for�vascular disease and dementia.
Alpha Lipoic Acid:� (ALA) is an essential cofactor in normal cellular metabolism and cellular�energy production.
Vitamin C and vitamin E: Can reverse symptoms caused by vitamin C and E�deficiencies.
As mentioned before, each patient is different and their lab work shows varying needs. However, with the Neural Zoomer Plus, we are able to get ahold of these symptoms, create a personalized treatment plan, and get them under control.�
As one can see, the data and knowledge we gain from these tests are truly eye-opening and give us an early advantage to help reverse or aid in prevention methods. We take the needs and concerns of every patient very seriously and work extremely hard to figure out the right method of treatment for them. Our goal is to help ensure that this lifestyle change is as smooth and easy on the patient as possible so they can get back to enjoying the activities they love and spending time with loved ones. The transition into a new lifestyle can be stressful, but with the information we gain from the tests, the knowledge we use from the doctor, and the willingness to change from the patient, we are set up to be the best team you can have to get your life back! – Kenna Vaughn, Senior Health Coach�
by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gut and Intestinal Health, Health, Wellness
In the last article, we talked about what the polyphenols do in the microbiome and in the previous section, we discussed about the microbiome functions in our bodies. However, today we will be concluding the three-part series of the microbiome functions in our bodies as well as presenting on the top 5 environmental toxins that can disrupt the gut microbiome, finding ways to de-stress ourselves, and learning about the different foods that can help detoxify our bodies so we can live a healthier life.
Top 5 Environmental Toxins Disrupting the Gut Microbiome
Triclosan
This is a synthetic antibacterial chemical found in personal care products such as soap, mouthwash, toothpaste, hand sanitizer, and deodorant. It is easily absorbed through the skin and gastrointestinal tract and rapidly alters the microbial composition of the digestive tract if it is ingested. However, this rapid restructuring of the gut microbiome impairs the immune system-regulating activities of gut microbes.
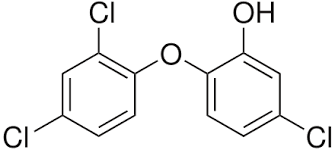
We use this chemical mostly in our daily skincare and hygiene routine so that way we won�t be sick. We tend to use this chemical compound to make us smell, look, and feel good frequently, especially in the cold and flu seasons where we use them the most so we won�t get sick. In fact, the frequent use of antibacterial products has been associated with an increased risk of food sensitivities, seasonal allergies, and asthma.
Pesticides

Surprisingly there are a staggering 1 billion pounds of pesticides used per year in the United States, and 5.6 billion pounds are used worldwide. Most farmers used it to spray down the insects so that way their crops won�t be destroyed. And we used pesticides on our lawns to get rid of weeds and keep the bugs off our gardens.
However, did you know that pesticides can kill beneficial bacteria in our gut? Studies, especially animal studies, indicate that pesticides can destroy the beneficial gut bacteria and can increase the risk of intestinal dysbiosis and cause immune system disorders, among with many other chronic health issues.
Plasticizers
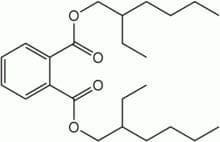
These are chemicals that provide flexibility or rigidity to plastic products. These chemicals are highly prevalent in our environment and have a significant impact on gut bacteria. �Surprisingly the most common plastics are mostly BPA (Bisphenol-A).

Bisphenol-A (BPA) can be found in plastic water bottles, receipts, and the lining of canned foods. They can alter the healthy gut flora and disrupts the body�s hormonal system by mimicking the hormone estrogen. We do use these to put our leftovers in after we consume food. But now and days when we meal prep our food, we do look for containers that are BPA- free. However, while often being marketed as �BPA-free,� the plastic alternatives may be equally, if not more, harmful to our gut microbes.
Bisphenol-S and bisphenol-F demonstrate endocrine-disrupting effects that are comparable to BPA. These adverse effects may extend to the gut microbiome, causing disruption. Phthalates are another class of endocrine-disrupting plasticizers that are used as solvents in personal care products and vinyl plastic, and they also reduce the levels of beneficial gut bacteria.
Heavy Metals
Heavy metals, such as cadmium, lead, and arsenic, can reduce the levels of beneficial bacteria in the gut that protect against intestinal inflammation and may promote inflammatory gastrointestinal disorders. All microbes are responsible for methylating or demethylating metals, and the exposure may exceed the capacity to perform this. Due to industrial pollution, heavy metals are the most common contaminants that are in the soil and drinking water when we grow food and drink from the tap.
Pharmaceutical Drugs
Surprisingly most pharmaceutical drugs can help our bodies fight off infections or alleviate some pains we may be inflicted. But those antibiotics can disrupt the gut microbiome and cause an imbalance to our gut bacteria. We here at Injury Medical clinic, actually recommend our patients to alternatives to these drugs if you don�t want to disrupt your gut microbiome.
Functional medicines like whole foods and supplements can actually alleviate the pains that may cause disruption in your body.
Protecting the Microbiome From Environmental Toxins
When you want to live a healthier life and want to protect your body�s microbiome try these alternatives to get rid of these environmental toxins.
- Instead of using conventional cleaning products, which often contain triclosan, try switching to a plant-based brand. Also, try making your own cleaning products at home with natural ingredients.
- Avoid commercial body care products, as these are a significant source of triclosan, phthalates, and parabens. If you have any absorption of these chemicals, try checking out the Environmental Working Group�s Skin Deep Cosmetics Database. This database can help you find natural, healthy body care products that don�t contain microbiome-disrupting chemicals.
- Eat organic produce. Conventionally-grown fruits and vegetables are a significant source of microbiome-disrupting pesticide exposure. Research indicates that consuming organic food can significantly lower your body burden of pesticides, thus protecting your gut microbes. But y9ou are going to eat organic produce, remember to wash it first to get rid of excess pesticides.
- Try reducing your plastic intakes and limit your consumption of canned foods to reduce your exposure to BPA and BPA alternatives. When you are meal prepping, try using glass or stainless-steel water bottles and storage dishes instead of plastic, and opt for fresh food instead of canned.
- Try filtering your drinking and bathing water. Unfortunately, tap water is rife with pesticide residues, heavy metals, plasticizers, and pharmaceutical drug residues and can come off as a milky white if it�s not treated. So try to consider investing in a high-quality water filter that can remove these substances from your drinking water.
- Support your gut microbiome by consuming prebiotics and probiotics. In a previous article, we talked about probiotics in our gut. Probiotics can add beneficial bacteria to your stomach and may even help in the metabolism of toxins that are in your body�s microbiome. Prebiotics, a form of indigestible dietary fiber, that feeds probiotics and helps to support their growth and proliferation in the gastrointestinal tract.
Other Forms of Whole Body Detoxification

There are many ways to try and detoxify our bodies, so here are some examples:
- Sauna therapy
- Yoga, trampoline
- Meditation
- Energy healing/shamanism
- Taking a much-needed vacation
- Learn communication methods to accommodate multiple needs and to deal with stressful situations
Rebuilding the Gut Microbiome
When local health coaches, practitioners, and chiropractors are helping patients, they can provide a comprehensive strategy to help them gain a healthier life. When you want to rebuild your gut microbiome, try to reconstruct the natural digestive function with food/herbals. This will help support the immune system and nutritional status by creating the good bacteria in your liver and flushing out the toxins out of your system. However, try to avoid any foods that can trigger inflammation and can cause leaky gut.
Rebuild Natural Digestive Function
When you are rebuilding your natural digestive function, try finding food and supplements that contain zinc, Vitamin C, and bitter greens that can aid in the production of hydrochloric acid (HCL). However, avoid excessive amounts of fat in your diet so you won�t cause a leaky gut Also take some enzymes if you need them until your digestive is balanced and fully restored.
Support the Immune System and Nutritional Status
When your immune system is being overworked, try using micronutrient testing to identify deficiencies. Most SIBO patients are typically low in B12/iron, zinc, magnesium, and vitamin D.� But all these vitamins and supplements can support the immune system. With SIBO patients, they try to work on cleaning out their liver since it�s one of the major organs that flushes out the toxins in our bodies. If you do have SIBO, try adding more fruits and plant foods that can help �clean out the liver.� Certain fruits can be tolerated and titrated up after treatment over time, but try to reduce meat/animal fats and fats in general; since they are harder to digest and can contribute to imbalanced bile acid secretion. Also, use liver support herbs and supplements such as glutathione and silymarin.
Avoid Foods that Can Provoke Inflammation

In a previous article, we talked about food sensitivity and what to do if you have it. Some testing can be helpful to determine if other foods may need to be eliminated. Here are the most common foods that provoke inflammation in dysbiosis are:
- Gluten
- Dairy
- Eggs
- Soy
- Corn
So if you have a food sensitivity, start by slowly build the natural SCFA�s with small amounts of natural resistant starch (e.g., cooked/cooled potatoes). However, if the patient is being treated with SIBO, introduce it after. Also considering adding other sources of food so it can help grow the good bacteria in your gut. But also keep HCL production active to clean out the stomach and upper part of the small intestine.
This will ensure that the good bacteria will grow over time with your diet and the help of probiotics and fermented food. But if a patient has SIBO take caution so the patient won�t disrupt the treatments they are in and are completed.
If you are taking care of a patient, carefully choose probiotic based on symptoms they have. Some will need a d-lactate free formula, and you can bring up the dosage over time until their treatment is complete. Some CFUs (colony forming units) will vary by product and viability through the GI tract (enteric-coated vs. not), and some probiotics may need to be used long term in some individuals.
Fermentation

Fermented foods are very beneficial to our gut flora as they actually help in the production of good bacteria in our intestinal barriers. Fermented foods and beverages are literally alive with strong pronounced flavor and nutrition. However, not all preserved foods are fermented with live cultures; some may be brined through the use of vinegar and/or salt, and do not impart probiotic benefit.
 �Fermentation is the transformation of food by various bacteria, fungi, and the enzymes they produce. It is important to recognize that fermentation is a natural phenomenon much broader than social, culinary practices; cells in our bodies are capable of fermentation. In other words, humans did not invent fermentation; it would be more accurate to state that fermentation created us.� � Dr. Alex Jimenez�D.C., C.C.S.T.
�Fermentation is the transformation of food by various bacteria, fungi, and the enzymes they produce. It is important to recognize that fermentation is a natural phenomenon much broader than social, culinary practices; cells in our bodies are capable of fermentation. In other words, humans did not invent fermentation; it would be more accurate to state that fermentation created us.� � Dr. Alex Jimenez�D.C., C.C.S.T.
Conclusion
So all in all, those are some of the many ways to actually help our bodies microbiome when we want to live a healthier life. Here at Injury Medical Clinic, local chiropractors and health coaches, actually use functional medicine to patients so that way, they can fix their ailments naturally, without the use of drugs and non-conventional methods. If we can change a person�s lifestyle with functional medicine, we can repair the microbes in our bodies, one at a time naturally, of course.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gut and Intestinal Health, Health, Wellness
In the last article, we talked about how the microbiomes in our body worked and functioned. As well as learning what each microbe does in our bodies but mostly in our gut. When we are learning more and more about the microbiome, we discover many exciting things that our bodies are capable of as well as being the workers in our intricate immune system. In today�s article, we will be taking a look at what polyphenols does to our microbiomes as well as specific vitamins that are very helpful to our gut and going in-depth more with SCFAs (Short Chain Fatty Acids) and the Tight Junction.
The Role of Polyphenols in Microbiome Balance
Polyphenols, or phenolic compounds, are considered a type of micronutrients, and they are plentiful in plants. They have been well-studied for their role in the prevention of chronic diseases such as CVD, cancer, and neurodegenerative diseases. They also have antioxidant properties, and there are several hundreds of polyphenols that are found in edible plants that serve a giant purpose of defending our bodies against ultraviolet radiation or aggression by pathogens.
To figure this out, think of it like this: The bacteria in our large intestine releases polyphenols from the plants we eat in our diets. It is then transformed into a diet composition that alters the bacterial ecosystem (through the prebiotic effects and antimicrobial properties) to make our gut happy.
Here are some of the microbes that are in polyphenols:
- Phenolic acids: These are derivatives of benzoic acid and derivatives of cinnamic acid.
- Flavonoids: These microbes contain flavonols (e.g., quercetin), flavones, isoflavones (e.g., phytoestrogens), flavanones, anthocyanidins, and flavanols (e.g., catechins and proanthocyanidins)
- Stilbenes: These microbes are resveratrol
- Lignans: these are minor in the human diet and are linseed oil
Surprisingly some factors affect the polyphenol content of plants, and these include:
- The ripeness at the time of harvest
- The environmental factors (exposure to light, soil nutrients, pesticides)
- processing and storage
When we eat organic fruits and vegetables, they have more polyphenol content that is usually, due to growing under slightly more stressed conditions. Which requires the plant to generate a stronger �defense and healing� response to the environment, and only 5�10% of the total polyphenol intake is absorbed in the small intestine. And 90-95% polyphenols that are linked to fibrous components must be liberated through hydrolysis by bacteria in the large intestine.
Surprisingly some polyphenols do not show up in plasma in humans after ingestion, and a large quantity is metabolized by intestinal bacteria or used to neutralize various pro-oxidizing agents in the intestinal lumen.
Clostridium and Eubacterium (which are both Firmicutes), are the primary metabolizers of polyphenols. Studies theorized that higher polyphenol intake may play a role in shaping the Bacteroidetes to Firmicutes ratio (e.g., inflammatory response potential, obesity, etc.) and can be harmful to our bodies.
However, more recent studies have shown effects of inhibition on Clostridium and Staphylococcus of polyphenols such as grape seed extract, in favor of Lactobacillus and other studies have demonstrated potent inhibition of phenolic compounds thymol (thyme) and carvacrol (oregano) on Escherichia, Clostridia and other pathogens, while simultaneously leaving Lactobacilli and Bifidobacteria have been unaffected.
Here are some other examples of some polyphenols:
- Resveratrol increases Clostridia, Lactobacillus, and Bifidobacteria
- Blueberry phenolics increase Bifidobacteria
- Phenolic compounds in tea suppress C difficile and C perfringens
- Catechins (found in high doses in teas and chocolate) act on different bacterial species (E. coli, Bordetella bronchiseptica, Serratia marcescens, Klebsiella pneumonia, Salmonella cholestasis, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis) by generating hydrogen peroxide and by altering the permeability of the microbial membrane
- Some studies have shown that polyphenols can interfere with bacterial cell signaling and quorum sensing (environmental sampling)
- Polyphenols can also cause bacterial populations to stop expansion through signaling interference
- Some research indicates certain polyphenols may be able to block the production of bacterial toxins (H. pylori and tea/wine polyphenols)
The Applications for A Diet

When it comes to eating a healthy diet, variety does matter. The colors, the types of fibers each organic food has, and whether you are going to do it daily or weekly. When you are trying to be in a healthy lifestyle, it always starts with the food. When you are looking for fresh produce, try to emphasize fresh, organic, and minimally processed versions of polyphenol-rich foods. However, don�t boil produce. Instead, try steaming then, and it is the best, but roasting or light frying is not only better, but it tastes so good.
Vitamins that help our Microbiome
When we are older, we tend to lose specific vitamins that actually helps us and our bodies to be healthier. Here are some of the vitamins that are really good for our gut and can help us prevent leaky gut.
Vitamin D
Vitamin D controls the development of gut-associated lymphoid tissue in our bodies. It is trafficking between gut dendritic cells, and they can differentiation of T-regs and T-reg function in our gut. But the expression of VDR, which influences IL (interleukin) production and tight junction integrity to help our gut.

When it comes to our gut, here are some of the effects of Vitamin D on the gut microbiome. The higher the Vitamin D levels are, they will allow commensal bacteria to secrete more AMPs (antimicrobial peptides). When patients take a high dose of Vitamin D, over 5 weeks can lead to a significant reduction in Pseudomonas spp and Shigella/Escherichia spp in upper gut intestines.
Another thing that Vitamin D does is that it can increase T cell differentiation in the colon. A lack of T-regs increased the incidence of asthma, allergies, autoimmune, and autism. But T-regs can prevent the development of aberrant immune responses such as autoimmune and food sensitivities. We here at Injury Medical Clinic, talk about functional medicine to our patients and try to help them recover from their ailments.
Because Vitamin D exposure fluctuates seasonally for many individuals, it has been observed that lower Vitamin D levels in the winter tend to lead to changes in the intestinal microbial balance. This will make our bodies have a decreased level of Bacteroidetes and an increased level of Firmicutes. This is the reason for �winter weight gain� in many individuals as F: B ratio changes.
Vitamin A

This is a retinoic acid that is required by dendritic cells (DCs) to induce T-cells (and B cells) which are the �tracking and regulation system� of the mucosal immune response. Because of this, T-cells must differentiate into T-regs to maintain a �calm and cool� system or immune tolerance to both our environment, symbiotic organisms, and food.
Omega-3 Fatty Acids

We talked about Omega-3s in a previous article as they are one of the many supplements that we can�t produce in our bodies. It can be mostly found in fish, and some plants can contain omega-3s. But it is a vital team player when we are trying to be healthy and can prevent a leaky gut. Not only that but omega-3s are crucial importance to more youthful skin.
SCFAs (Short Chained Fatty Acids)
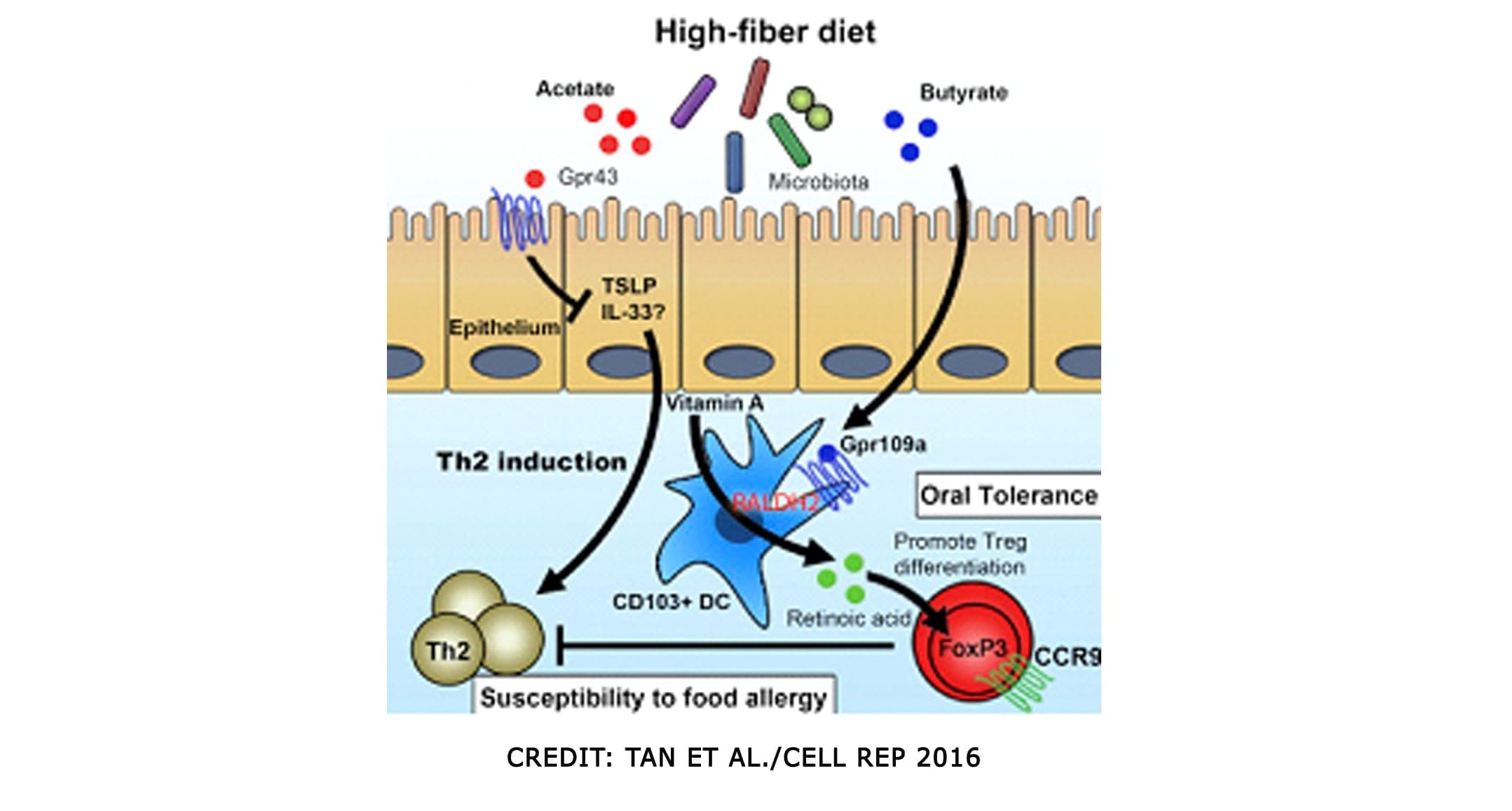
SCFAs (Short Chained Fatty Acids)�are well-studied to demonstrate anti-inflammatory properties in the large intestine. They are the primary source of fuel for cells lining the intestinal epithelium of the large intestine. They contained: �Butyrate, Proprionate, Acetate. In a previous article, we discussed what SCFAs do when we eat fatty food. It can be both good or bad, depending on what kinds of food you consume. SCFAs act on G-protein coupled receptors to induce differentiation of T-cells, but also on those GPRs in DCs. They can both be direct and indirect influences on our gut.
SCFAs can produce bacteria and can directly impact T-reg production. And that SCFAs inhibit the mucosa and competitively inhibit opportunists. Some foods that provide higher resistant starch will typically yield the most short-chain fatty acids upon microbial fermentation
Tight Junction Modulations
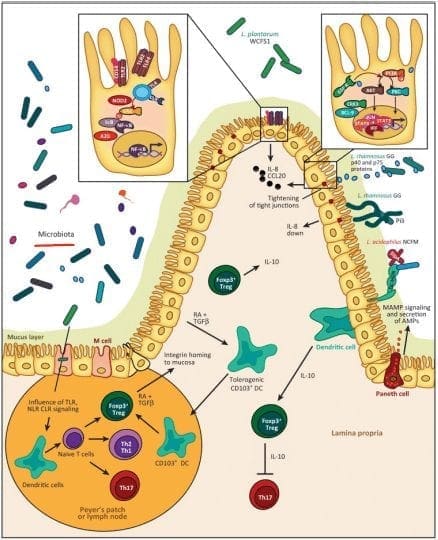
The tight junctions are the gateways between the epithelial cells. In a previous article, we took a look at what the tight junction is. They control the flow of nutrients, macromolecules, and other substances that are usually allowed to pass through without cellular diffusion or absorption.
Conclusion
All in all, we covered a lot of information about what polyphenols does as well as specific vitamins and supplements that can help our bodies prevent a leaky gut. The microbiomes in our collection and the use of functional medicine can be beneficial in helping us not only to a better, healthier life but, a working, functional body for us when we are older. Tomorrow we will end this three-part series with foods and tips to have a healthy microbiome in our gut and our bodies.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gut and Intestinal Health, Health, Wellness
In the last three articles, we introduced the wheat zoomer, went in-depth about the intestinal permeability, and the microbes in our gut. We also talked about the hidden problem with gluten as well. Today we will be discussing what to do after your patient comes in for a check-up after completing their gut healing process. For this to work, patients have to be gluten-free for the results to work. After the healing phase, local chiropractors, health coaches, and physicians can introduce gluten back slowly into the patient’s diet.
Checkups
When the patient comes in for a check-up, here are some of the things doctors look for:
- If everything is green, re-introducing gluten may be possible for patients.
- If anything is still yellow or red, patients must continue to avoid gluten and keep the gut healing process until the test results are all green.
After the appointment, patients can retest the wheat zoomer in about 3-9 months. However, there are about 50% of individuals with celiac disease that may also have antibodies to casein and may have to go dairy-free to heal their gut. Although some individuals are gluten sensitive may not be lactose intolerant but can go dairy free if they want to.
Lectins
Surprisingly all plants have lectin, but some of them are not problematic to humans thankfully. Legumes and grains play a role in increasing the diversity of microbes that may be beneficial or harmful to our bodies. Wheat Germ Agglutinin is the only specific lectin to the Wheat Zoomer, but it doesn�t reflect the overall lectin sensitivity but, consider the lectin zoomer to determine any particular lectins that your patient is sensitive to without eliminating unnecessary foods that are beneficial to the gut healing process.
Soy

Many studies show that soy may be a problem due to the saponin since it does have higher lectin count. Here at Injury Medical clinic, we suggest that running the Lectin Zoomer on our patients with intestinal permeability makes sense for them to heal correctly. But animal studies stated that soybean agglutinin has the effect of the increasing release of zonulin away from the TJ (tight junction). And a human study reported that if the soy saponin is poorly absorbed or utilized by the human intestinal epithelial cell will end up metabolizing the intestinal bacteria and cause more harm to human.
Surfactants

Known as sucrose monoester fatty acids is mostly used in cosmetics, food preservatives, food additives. Sucrose esters are used as a surface treatment on some fruits like peaches, pears, cherries, apples, and bananas. It keeps the moisture on the peel or rind controlled. Sucrose mono ester used in cosmetics as an emulsifier.
Bone broth/Collagen

Up to this date, there are minimal studies that have examined the role of bone broth or collagen that it repairs the intestinal barrier. It all depends on the animal and the bone it comes from and the contents that people have put in a bone broth soup.
However, there is a small body of evidence for the use of collagen peptides seem to support the usage in their autoimmune disease treatment protocol. But the results cant be extrapolated whether bone broth would have similar effects. So more studying about bone broth is needed.
Organic Produce

When people say that they are going organic, it is beneficial for people that are willing to change to a healthy lifestyle. Granted that organically produces are locally grown, but still, organic produce or non-organic produce will not have less or safer pesticides. They are different, but all of the crops like fruits and vegetables are grown with pesticides.
Just like all fruits and vegetables, still, wash them thoroughly to reduce the number of pesticides and surfactants exposure and enjoy them to your heart’s content.
Resistant Starches

Resistant starch foods are a form of carbohydrates that can be converted to short fatty acids by SCFA-producing bacteria. With higher SCFA levels, these starches aid immune tolerance, T-cell differentiation, and intestinal barrier homeostasis. Other types of fibers are high value, and variety is essential. Like for example, cooked/cooled rice and potatoes are excellent resistance starch foods.

“Eat like a vegetarian who eats meat.”- Dr. Alex Jimenez�D.C., C.C.S.T.
Polyphenol Foods
Polyphenols, flavonoids and other phenolic compounds will aid to maintained TJ stability through inhibiting phosphorylation of TJ complexes.
Fermented Foods

Fermented food or drinks that contain live probiotic cultures are excellent in promoting a healthy gut. However, it is challenging to study fermented food and beverages due to highly variable stains, composition, nutrient content. But studies found that participants who drink a fermented plant extract drink saw improvements in their body and increased total antioxidants and total phenolic in plasma as well as with reducing total C and LDL-C.
Beneficial bacteria like Bifido and Lactobacillus were increased while E Coli and C perfringens were decreased in the gut. So drinking or eating fermented food can help our gut and help our bile growth to be flushed out.
Granted, we all know that trying to be healthy is very hard. It is true that even though exercising is easy because we can do it over and over again until we are masters at it but, when we overdo the exercises it will cause harm to our bodies and hurting it in the process. �Eating healthy is hard as well because our bodies may have a food allergen or a food sensitivity that will make us disappointed that we can�t enjoy the foods we want to eat. Yes, eliminating diets are very hard and challenging to follow in the long term and have poor compliances when we don�t put in the work. So start by exploring other tests to tailor patients diets to meet their health needs and prolong their recovery period.
Supplements

Supplements are fantastic to aid the valuable nutrients and minerals our bodies can�t produce. Common supplements prescribed include:
- L-glutamine
- Vitamin D3
- Collagen
- Colostrum
- Zinc carnosine
- Ox bile
- Omega-3s
- Turmeric/Quercetin
- Magnesium
Stress Management

Granted that stress is part of our lives and we can manage small amounts of it, and it is beneficial to us, but some people have chronic stress and have to go to a doctor to get it treated. But there is hope as there are ways to manage stress and doing it functionally and naturally.
Conclusion
So all in all, just to recap, if your patient is good to go and their test results are all green, you can introduce gluten back to their diets again in small increments and can be rested in about 3 to 9 months. But if your patient does have a gluten allergen then just let them know that they must continue to be gluten-free. We here at Injury Medical Clinic, always put our patient�s needs first for them to live their best lives with functional natural medicine.
by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gut and Intestinal Health, Health, Wellness
With the last article, we talk about how our gut system actually works. With the many microbes that inhabit our intestines, we do try to our best to lead a healthier lifestyle. Here at Injury Medical, local chiropractors and health coaches inform our patients about functional medicine as well as helping them to prevent a leaky gut. Here we will talk more about what the microbiomes in our intestines do when we are exposed to harsh environments.
The Microbiome
The significant role of the microbiome in the epithelial barrier integrity and breakdown. However, we can�t have a conversation with patients about intestinal permeability and food sensitivities without telling them about the role the microbiome plays.
The Wheat zoomer is rich with data but adds the Gut zoomer with the patients; the results are more accurate.
Microbial Influence on our Intestines
- Immune influence: One of the leading roles that microbiomes play in the immune system is that it generates byproducts of carbohydrates/fiber fermentation that will influence T-cell differentiation. Without the distinction, we will see an increase of being at high risk of autoimmune diseases, allergies, autism, and asthma.
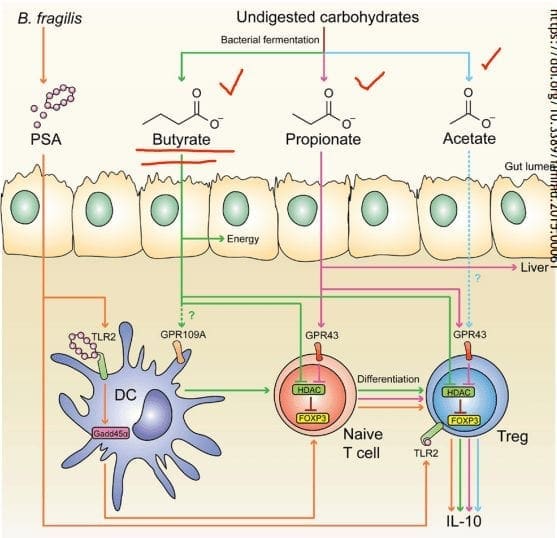
- SCFAs (Short Chain Fatty Acids): The food we consume gets fermented for good bacteria to feed on. SCFAs creates fermented fibers by commensal microbes into Butyrate, Propionate, Acetate. These three are essential to the intestinal immune system. These SCFAs can influence T-cells differentiation differently, but it still gets the same results.
- T-cell Differentiation: na�ve T-cells that activate the immune response to T-regs (police cells) to signal B-cells, and it can be a good thing. But if the T-cells activate and differentiate the wrong cells, it will cause inflammation.
When T-cells differentiation is less abundant, there will be a higher incidence of food sensitivities, autoimmune disease, asthma, and allergies. But when there is an abundance of butyrate, the patients have lower rates of colon cancer and colitis.

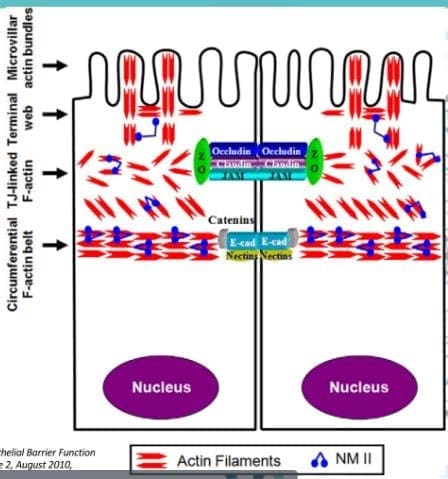
- Tight Junction: Lactobacillus plantarum and Lactobacillus rhamnosus are the reinforcers for the tight junction while inducing TLR (toll-like receptors) outside the intestinal epithelial walls, as well as increasing the abundance of zonulin-occludin into the tight junction.
SCFAs also play a vital role in the tight junction by lessening the extent propionate, inducing LOX (lipoxygenase) activity and increasing tight junction�s stability while reducing permeability.
Pathogens/Pathobionts: Can be an influence in the epithelial barriers as they can be opportunistic or conditionally pathogenic. Various pathogens like enteropathogenic E.coli can alternate the tight junction�s system. However, if there is a low abundance of L. plantarum, then it will lead to infections and disruption as well as disorganization of the actin cytoskeletons. This can be reversed by incubating the epithelial cells with L. plantarum to create a high density of actin filaments to the tight junction and repair itself.
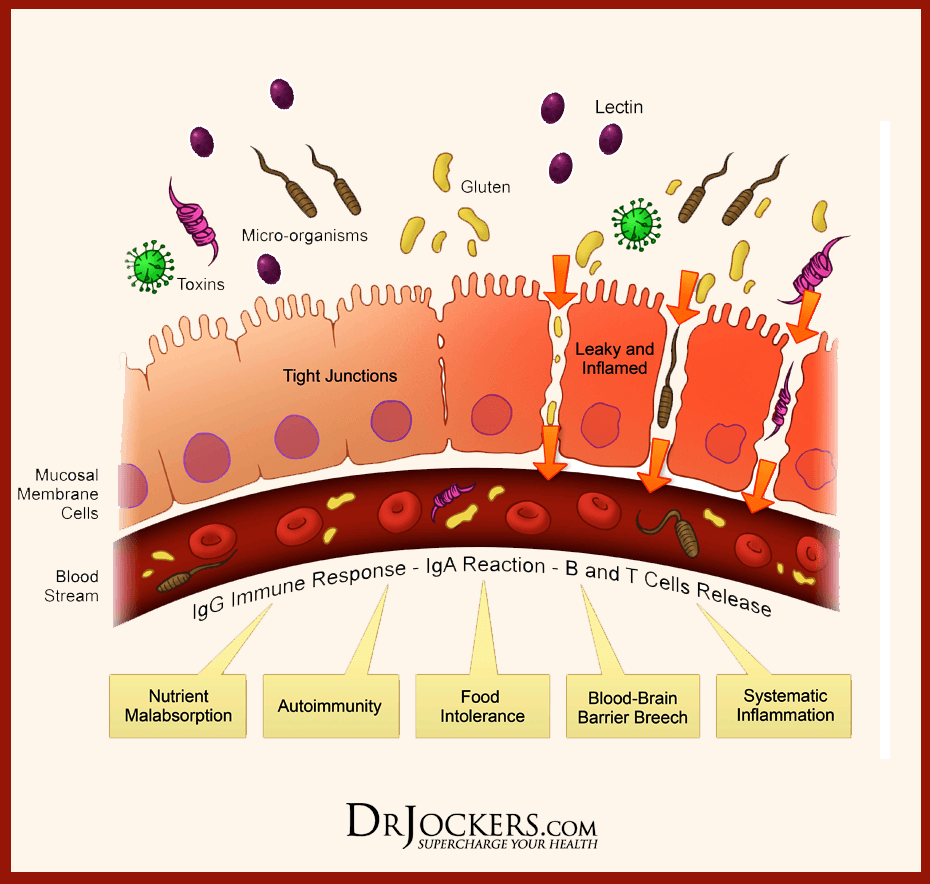
- Zonulin, actin, and LPS: In the previous article, zonulin is the �gatekeeper� proteins that are responsible for opening and closing the tight junction. We talked about how if there is a low count of zonulin, it can cause inflammation, but if the zonulins are high, they can increase the IP and may facilitate enteric translocation by disassembling the tight junction. With less zonulin, it can be an overgrowth of b bacteria cells, thus causing more inflammation.
Actins are the structural and functional cells in the tight junction. However, if bacteria enter the actin cell walls, the bacteria will release toxins to the cell walls, it not only damages it but causes it to leak as well. This will make the damage actin cells not only paracellular but also intracellular to the damage actin cell walls.
Actin walls can also be affected when surfactants are involved. Surfactants are food agents and are known to affect the absorption of food substances in the gastrointestinal tract. They are not problematic, but when there is a low count on TEER, it can increase permeability and disband the tight junction.
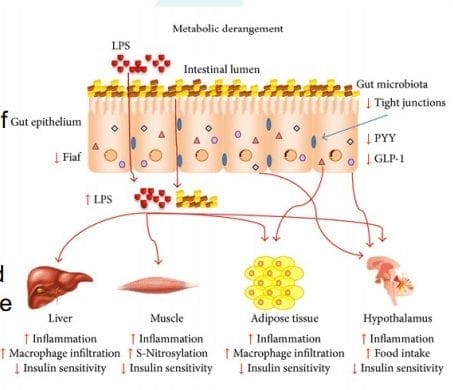
LPS (Lipopolysaccharide) acts as a barrier and is recognized by the immune system as a marker for detecting bacterial pathogen invasion. It�s responsible for the development of inflammatory response in our gut.
Diet and Lifestyle
Diet and lifestyle contributions to the epithelial barrier integrity and breakdown. With the Wheat and Gut Zoomer helping out our intestinal barriers. Specific diets and lifestyles can also play effect to what is causing discomfort to our gut. These factors can cause our gut to be an imbalance, gastro discomfort, inflammation on our intestinal epithelial barriers.
- Gluten: Gliadin is the main peptide that can cause gluten sensitivity. The gliadin protein can bind with many microbes, causing discomfort to our intestines and gut. Plus giving us an autoimmune disease, skin allergens, and chronic illnesses.

- Keto/High Fat Intake: Increase fat meals cause an increase of permeability, and if a patient has a high gram-negative, it will cause problems. But it can be beneficial, to those who don�t have the gram-negative bacteria in their system but, certain microbes like SCFAs do cling onto these fatty substances. In order to give patients an accurate result use both the Gut Zoomer and Wheat Zoomer to better the chances. Higher fat meals suppress beneficial bacteria. Causing a double risk of toxins in the bloodstream as well as inflammation.

- Alcohol: Patients are more willing to give up alcohol than gluten. Alcohol can be a stress reliever but can lead to addiction. It can be one of the causes of redistribution of the junctional proteins. One glass of wine a day is ok, but some patients don�t see alcohol as a mediator for reducing problems.
- Lectins: Lectins are contributors to permeability and impair the integrity of the intestinal epithelial layer, allowing passage through. Antibiotics for WGA can help lower the permeability of the intestinal wall barriers.

- Stress: Stress can cause discomfort and permeability in the intestinal epithelial barrier from high levels of cortisol.
Conclusion
Yes, gluten can cause inflammation in the intestinal epithelial barriers, but many factors that we discussed are also factors that can cause physiological assaults on the barrier�s integrity and stability of the intestinal ecosystem. Dr. Alexander Jimenez informs our patients about the importance of how functional medicine works with the combination of the Gut Zoomer and the Wheat Zoomer. This is not only protecting our gut but by giving us the information on what we can do to prevent a leaky gut.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Gut and Intestinal Health, Health, Wellness
Today local chiropractors will be giving a description of the wheat zoomer. We will be giving a brief description of each panel, its markers, and the basic interpretations of the test. We will also be discussing the considerations for the patients and providers before we take The Wheat Zoomer test.
What is a Wheat Zoomer test?
The Vibrant wheat zoomer has 6 test in one to identify if the patient has wheat and gluten sensitivity. The Vibrant wheat zoomer does give our patients a thorough evaluation and we ask our patients if they started to be gluten-free or was gluten-free, either from birth or not and how much gluten-contained food did they eat. One of the best ways to ensure that our patients may have a gluten sensitivity is that if they have a food diary for us to look over and that way we can determine how severe of the wheat zoomer.

IgA vs IgG
In order for us to know about the wheat zoomer in our patient�s body, we must know about the immunoglobulins. The first one is IgA. IgA immunoglobulins are mucosal and are found primarily in the epithelial lining of the body: intestinal tract, lungs esophagus, blood-brain barrier and around internal organs. They are:
- The first line of defense.
- More accurate to our gut.
IgG immunoglobulins found in the blood system and are numerous in the body They are considered �systemic� and are non-specific to any one location. Not all IgG antibodies are sensitive though, some of them can indicate that an antigen has �leaked� into the blood and the immune system tagged that antigen as a �non-self�. And they are not diagnostic as IgG+IgA, but if IgA is absent, the antibodies are more relevant.
- If the patient is recently gluten-free, the antibodies will tell us that the antigen hasn�t cleared out in the patient�s system from past weeks of eating gluten.
Celiac
Celiac is a growing autoimmune disease, about 1% of the population is affective and 1 in 7 Americans have a reaction to wheat or wheat gluten disorder. The Vibrant test can determine a 99% sensitivity and 100% specify on the celiac antibodies.
- Total IgA and Total IgG measure both the IgA and IgG to determine the patient�s reactivity to gluten
- Cut off for IgA is 160 as well as a bottom 1/3rd
- Not all traditional markers for celiac disease doesn�t need to be elevated if tTg2 is elevated.
Intestinal Permeability
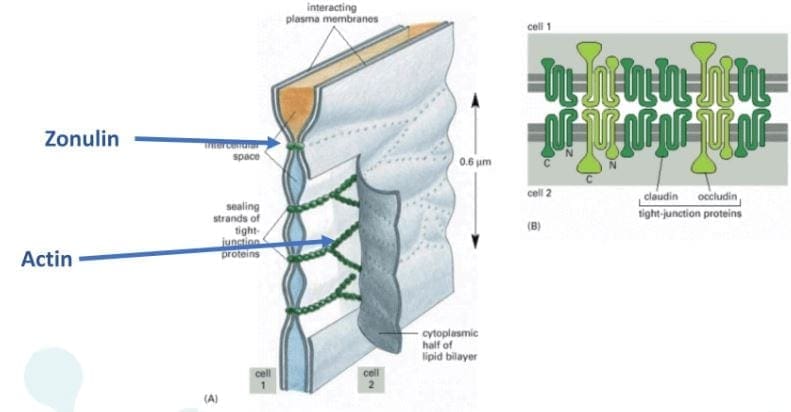
Zonulin is the gatekeeper for the intestines and controls nutrient flows and molecules across the membrane. It is a protein complex inside the intestinal tight junctions and can be increased by either gluten and high-fat meals.
Anti-Actin, especially f-Actin is in the smooth muscle of the intestines. Actin is part of the actomyosin complex. Vibrant can isolate f-Actin to get a more accurate picture of the patient�s immune response to the intestines. While antibodies in actin can identify intestinal destruction and indicate autoimmune diseases like connective tissue disease and autoimmune hepatitis.
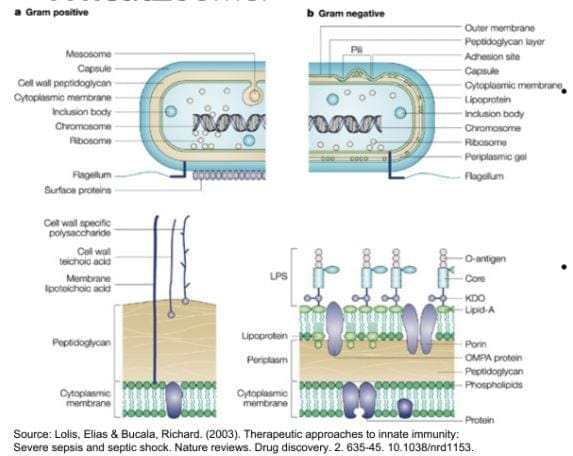
Lipopolysaccharide (LPS) is produced by gram-negative enterobacteria. It is very potent and can cause inflammation. Plus it�s one of the indications of a leaky gut. Practitioners can draw additional lab test for cardiovascular, inflammatory markers, and diabetes/insulin resistance.
Here at Injury Medical Clinic, we suggest to our patients to try a Vibrant GutZoomer to identify the source of their ailments before we add the Vibrant WheatZoomer.
Gluten-mediated Autoimmunity
Fusion Peptide is the new addition to Wheat Zoomer in 2017. It is cross-linked to tTg and can identified celiac progression from 14 months to 4 years.
Differential Transglutaminases can detect autoimmune reactions to gluten that are not celiac or are becoming celiac. However, gluten is still a trigger but react differently in the celiac autoimmune disease such as:
- Transglutaminase 3= skin manifestations of autoimmunity like dermatitis herpetiformis, eczema, and psoriasis.
- Transglutaminases 6= neurological manifestations of autoimmunity in the cerebellum like gluten ataxia, gate abnormalities, balance and coordination issues.
Wheat Germ Agglutinin
Wheat Germ Agglutinin is the lectin component of wheat but, it is not a component to gluten. Dr. Jimenez can detect a patient’s low level of Vitamin D absorption from the patient�s results. And Wheat Germ Agglutinin is commonly used as an additive in supplements and the supplement can still be called gluten-free due to the different protein structure.
Gliadin, Glutenin, and Prodynorphin
Gliadin and glutenin are what makes up the super protein in gluten. Most people are reacting to the Gliadin portion of gluten and gliadin binds with tTg2 in celiac and binds zonulin to a leaky gut in patients. Gliadin reacts to any antigens can indicate a sensitivity to gluten in patients and gluteomorphin are peptides in wheat and react as a euphoria receptor to the brain. Prodynorphins antibodies can indicate that gluten reacts to signaling hormones and affect the patient’s mood.

Sadly though, patients do have a hard time withdrawing gluten in their diet since their antibodies are used to the compound and it up to us, here at Injury Medical Clinic to gently push our patients to have the will power to fix what is causing them to have ailments.
Wheat Allergin
Wheat Allergen is the true allergen body. Some patients that already know that they are allergic to wheat from a young age but it doesn�t decrease when wheat is eliminated and can remain long term after the allergic response happens.
Glutenin
Glutenin is the other part of the gluten compound. However it is less common to some people, but some individuals do show reactivity to glutenin, thus still have a gluten sensitivity. But there is no clinical difference to the reactivity to glutenin from high to low molecular weight.
Non-Gluten Wheat Proteins
Surprisingly Vibrant has an advantage to their test as they have a panel for patients that don�t have a gluten sensitivity but a wheat sensitivity. The Vibrant advantage to the unique non-gluten wheat panel shows us that:
- Proteins in wheat unrelated to gluten but relevant to immune reactions.
- It is 30% of the protein molecular weight of wheat.
- Some individuals are more reactive to wheat proteins than gluten itself.
If they are trying to be gluten-free, patients still have to read the labels to see if any hidden wheat starches are in the ingredients. But not all food products are gluten-free if they have the wheat protein in them.
Conclusion
If the patient is trying to be gluten-free but previously ate gluten compound food. They can still feel the reaction if they discovered that they have a sensitivity to gluten by their practitioner. And must take precautions when they are reading the labels of the products they are going to buy and consume. In the next four articles, we will discuss what the Wheat Zoomer can provide as well as, discussing about what causes leaky gut, what actually goes on in our patient�s intestines, and wrapping up on what to do after the Wheat Zoomer heals and restores the gut barrier.

















 �Fermentation is the transformation of food by various bacteria, fungi, and the enzymes they produce. It is important to recognize that fermentation is a natural phenomenon much broader than social, culinary practices; cells in our bodies are capable of fermentation. In other words, humans did not invent fermentation; it would be more accurate to state that fermentation created us.� � Dr. Alex Jimenez�D.C., C.C.S.T.
�Fermentation is the transformation of food by various bacteria, fungi, and the enzymes they produce. It is important to recognize that fermentation is a natural phenomenon much broader than social, culinary practices; cells in our bodies are capable of fermentation. In other words, humans did not invent fermentation; it would be more accurate to state that fermentation created us.� � Dr. Alex Jimenez�D.C., C.C.S.T.




































