
Functional Neurology: What You Need to Know About Obesity and Depression
Doctors understand that people with depression can experience weight gain and over time, it may eventually lead to obesity if left untreated. Depression is also associated with poor eating habits, overeating, and a more sedentary lifestyle. According to the Centers for Disease Control and Prevention (CDC), approximately 43 percent of people with depression have excess weight or obesity. In a 2002 research study, scientists found that children with depression had an increased risk of suffering from obesity. In the following article, we will discuss what you need to know about obesity and depression. �
Understanding Obesity and Depression
Mental health issues, such as anxiety and depression, are associated with obesity. A 2010 research study found that about 55 percent of people with obesity had an increased risk of developing depression and other mental health issues compared to “healthy” people. Moreover, obesity can also cause a variety of other health issues, including joint pain, hypertension, and diabetes, among others. Anxiety, by way of instance, can also ultimately cause depression and obesity. Scientists believe that stress can make people turn to food as a coping mechanism. This can eventually lead to excess weight gain and obesity. �
Scientists were once hesitant to connect obesity and depression, however, further evidence from numerous research studies has demonstrated that excess weight or obesity can cause a variety of mental health issues like anxiety and depression. Many doctors utilize a multi-pronged treatment approach to help improve a patient’s mental and physical health. Scientists still don’t quite understand how obesity is closely associated with depression but it is clear that there’s a connection between obesity and depression. Furthermore, research studies demonstrated that mental health issues may also cause obesity. �
The Connection Between Obesity and Depression
Obesity and depression, as well as any other mental health issues, are can also cause a variety of other health issues if left untreated, including chronic pain, coronary heart disease, hypertension, sleep problems, and diabetes. Fortunately, all of these health issues can be properly diagnosed, treated, and prevented by following a proper treatment program. Treating the underlying source of a patient’s depression, by way of instance, may help restore their energy in order to help them participate in exercise and physical activities. Engaging in exercise and physical activities may, in turn, help patients lose weight. �
Dietary and lifestyle modifications can also be utilized to help treat a variety of mental and physical health issues, including obesity and depression. It’s essential to seek immediate medical attention from qualified and experienced doctors who can help guide patients in the right direction. If you’ve ever experienced any of the following red-flags, symptoms, or side-effects, including loss of all interest in regular activities that you used to enjoy, an inability to get up from bed or leave your house, abnormal sleep patterns, feeling tired or fatigued, and weight gain, talk to your doctor about what you can do. �
Dealing with Obesity and Depression
A strategic treatment plan for obesity and depression can ultimately be different, however, several methods and techniques can also help improve the underlying source of the other health issue. You can reduce your risk of developing obesity and depression by following proper nutritional or dietary guidelines and engaging in exercise or physical activities. Participating in exercise or physical activities is a great way to naturally help boost endorphins as well as neurotransmitters like dopamine and serotonin that help boost and balance mood, ultimately helping you lose weight and feel better. �
Research studies demonstrated that engaging in exercise or physical activities at least once per week can have a considerable effect on symptoms of depression. Doctors also understand that when you have depression, finding the motivation to participate in exercise or physical activities can be challenging. Doctors recommend taking small steps, such as engaging in 10 minutes of exercise or physical activities every day, may help people get in the habit of participating in exercise or physical activities. Talk to your doctor about the right amount of exercise or physical activity that you should do. �
Talking to a therapist or psychologist is a well-known treatment approach for a variety of mental and physical health issues. From anxiety and depression to excess weight and obesity, a therapist or psychiatrist can help you process the emotional factors that may be causing the underlying source of your health issues. They can also help you embrace changes that will help you improve your quality of life. Following a strategic treatment plan and always being honest with your healthcare professional may ultimately help improve obesity and depression as well as any symptoms, side-effects, and complications. �
Obesity and depression are well-known health issues that need long-term care and attention. It�s essential to talk to your doctor regardless of whether you�re following your strategic treatment plan. Being honest about what you are and aren�t doing is the only way for your doctor to understand and help with your underlying health issues. Your doctor is your best resource for information and they�ll work with you to find the best treatment for your needs, help you create a healthier lifestyle, and hold you accountable for the changes you seek. People with obesity and depression can ultimately restore their wellness. �

Research studies demonstrated that obesity is associated with mental health issues like anxiety and depression. Doctors understand that people with depression can experience weight gain and over time, it may eventually lead to obesity if left untreated. Depression is also associated with poor eating habits, overeating, and a more sedentary lifestyle. According to the Centers for Disease Control and Prevention (CDC), approximately 43 percent of people with depression have excess weight or obesity. In a 2002 research study, scientists found that children with depression had an increased risk of suffering from obesity. In the following article, we will discuss what you need to know about obesity and depression, including the connection between obesity and depression as well as dealing with these mental and physical health issues, among others. – Dr. Alex Jimenez D.C., C.C.S.T. Insight
Doctors understand that people with depression can experience weight gain and over time, it may eventually lead to obesity if left untreated. Depression is also associated with poor eating habits, overeating, and a more sedentary lifestyle. According to the Centers for Disease Control and Prevention (CDC), approximately 43 percent of people with depression have excess weight or obesity. In a 2002 research study, scientists found that children with depression had an increased risk of suffering from obesity. In the article above, we will ultimately discuss what you need to know about obesity and depression. �
The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues or functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or disorders of the musculoskeletal system. Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900.�
Curated by Dr. Alex Jimenez �
References:
- Holland, Kimberly. �Are Obesity and Depression Related? And 9 Other FAQs.� Healthline, Healthline Media, 11 May 2018, www.healthline.com/health/depression/obesity-and-depression.
Neurotransmitter Assessment Form
The following Neurotransmitter Assessment Form can be filled out and presented to Dr. Alex Jimenez. The following symptoms listed on this form are not intended to be utilized as a diagnosis of any type of disease, condition, or any other type of health issue. �
Additional Topic Discussion: Chronic Pain
Sudden pain is a natural response of the nervous system which helps to demonstrate possible injury. By way of instance, pain signals travel from an injured region through the nerves and spinal cord to the brain. Pain is generally less severe as the injury heals, however, chronic pain is different than the average type of pain. With chronic pain, the human body will continue sending pain signals to the brain, regardless if the injury has healed. Chronic pain can last for several weeks to even several years. Chronic pain can tremendously affect a patient’s mobility and it can reduce flexibility, strength, and endurance. �
Neural Zoomer Plus for Neurological Disease
Dr. Alex Jimenez utilizes a series of tests to help evaluate neurological diseases. The Neural ZoomerTM Plus is an array of neurological autoantibodies which offers specific antibody-to-antigen recognition. The Vibrant Neural ZoomerTM Plus is designed to assess an individual�s reactivity to 48 neurological antigens with connections to a variety of neurologically related diseases. The Vibrant Neural ZoomerTM Plus aims to reduce neurological conditions by empowering patients and physicians with a vital resource for early risk detection and an enhanced focus on personalized primary prevention. �
Food Sensitivity for the IgG & IgA Immune Response
Dr. Alex Jimenez utilizes a series of tests to help evaluate health issues associated with a variety of food sensitivities and intolerances. The Food Sensitivity ZoomerTM is an array of 180 commonly consumed food antigens that offers very specific antibody-to-antigen recognition. This panel measures an individual�s IgG and IgA sensitivity to food antigens. Being able to test IgA antibodies provides additional information to foods that may be causing mucosal damage. Additionally, this test is ideal for patients who might be suffering from delayed reactions to certain foods. Utilizing an antibody-based food sensitivity test can help prioritize the necessary foods to eliminate and create a customized diet plan around the patient�s specific needs. �
Gut Zoomer for Small Intestinal Bacterial Overgrowth (SIBO)
Dr. Alex Jimenez utilizes a series of tests to help evaluate gut health associated with small intestinal bacterial overgrowth (SIBO). The Vibrant Gut ZoomerTM offers a report that includes dietary recommendations and other natural supplementation like prebiotics, probiotics, and polyphenols. The gut microbiome is mainly found in the large intestine and it has more than 1000 species of bacteria that play a fundamental role in the human body, from shaping the immune system and affecting the metabolism of nutrients to strengthening the intestinal mucosal barrier (gut-barrier). It is essential to understand how the number of bacteria that symbiotically live in the human gastrointestinal (GI) tract influences gut health because imbalances in the gut microbiome may ultimately lead to gastrointestinal (GI) tract symptoms, skin conditions, autoimmune disorders, immune system imbalances, and multiple inflammatory disorders. �
Formulas for Methylation Support
� XYMOGEN�s Exclusive Professional Formulas are available through select licensed health care professionals. The internet sale and discounting of XYMOGEN formulas are strictly prohibited.
Proudly,�Dr. Alexander Jimenez makes XYMOGEN formulas available only to patients under our care.
Please call our office in order for us to assign a doctor consultation for immediate access.
If you are a patient of Injury Medical & Chiropractic�Clinic, you may inquire about XYMOGEN by calling 915-850-0900.
For your convenience and review of the XYMOGEN products please review the following link. *XYMOGEN-Catalog-Download �
* All of the above XYMOGEN policies remain strictly in force. �
� �
Modern Integrated Medicine
The National University of Health Sciences is an institution that offers a variety of rewarding professions to attendees. Students can practice their passion for helping other people achieve overall health and wellness through the institution’s mission. The National University of Health Sciences prepares students to become leaders in the forefront of modern integrated medicine, including chiropractic care. Students have an opportunity to gain unparalleled experience at the National University of Health Sciences to help restore the natural integrity of the patient and define the future of modern integrated medicine. �








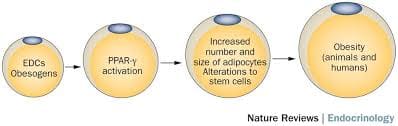
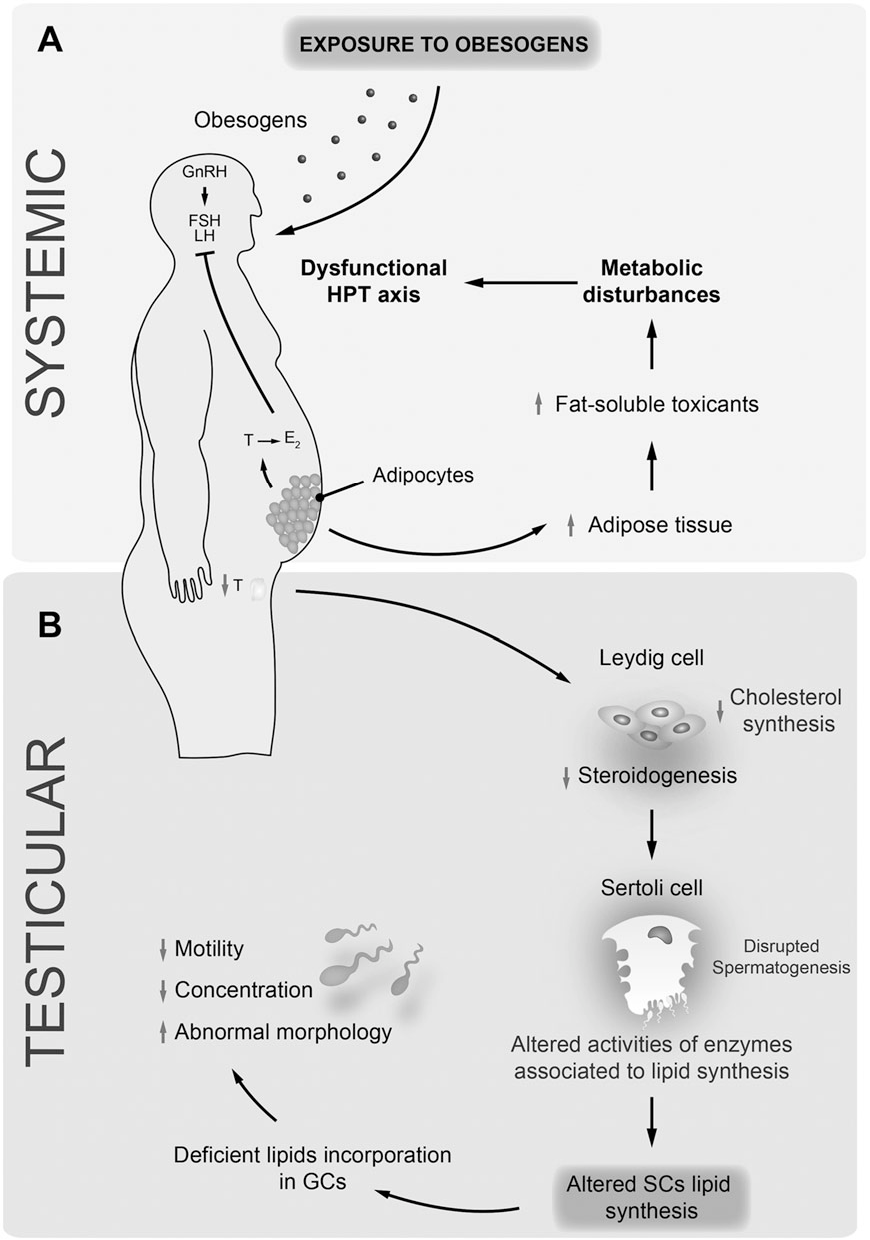
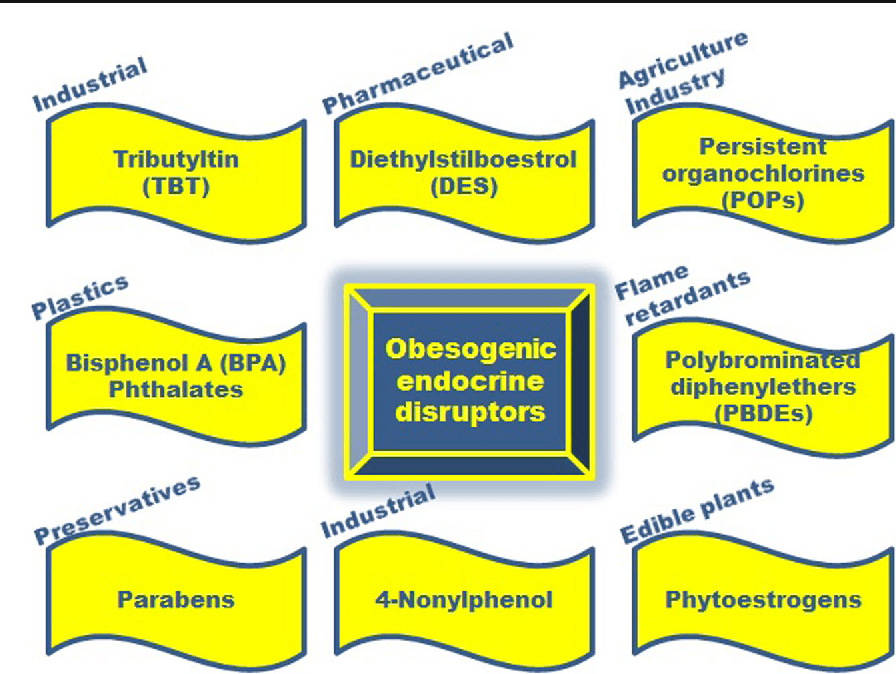
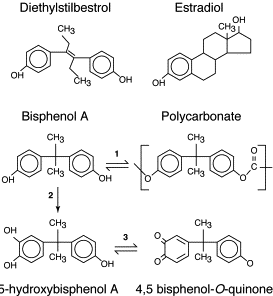





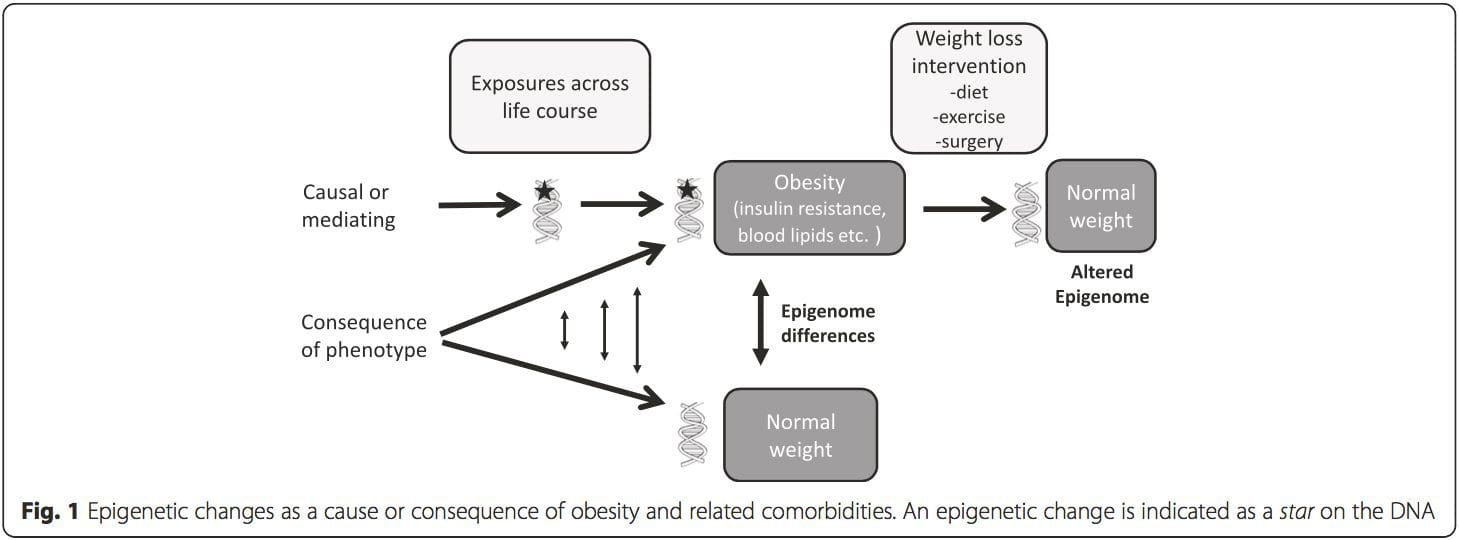
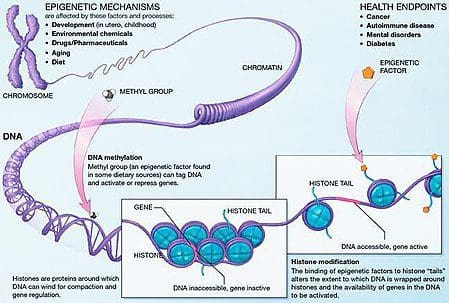 Obesity is a complex, multifactorial disease, and better understanding of the mechanisms underlying the interactions between lifestyle, environment, and genetics is critical for developing effective strategies for prevention and treatment [1].
Obesity is a complex, multifactorial disease, and better understanding of the mechanisms underlying the interactions between lifestyle, environment, and genetics is critical for developing effective strategies for prevention and treatment [1]. Animal models provide unique opportunities for highly controlled studies that provide mechanistic insight into�the role of specific epigenetic marks, both as indicators of current metabolic status and as predictors of the future risk of obesity and metabolic disease. A particularly important aspect of animal studies is that they allow for the assessment of epigenetic changes within target tissues, including the liver and hypothalamus, which is much more difficult in humans. Moreover, the ability to harvest large quantities of fresh tissue makes it possible to assess multiple chromatin marks as well as DNA methylation. Some of these epigenetic modifications either alone or in combination may be responsive to environmental programming. In animal models, it is also possible to study multiple generations of offspring and thus enable differentiation between trans-generational and intergenerational transmission of obesity risk mediated by epigenetic memory of parental nutritional status, which cannot be easily distinguished in human studies. We use the former term for meiotic transmission of risk in the absence of continued exposure while the latter primarily entails direct transmission of risk through metabolic reprogramming of the fetus or gametes.
Animal models provide unique opportunities for highly controlled studies that provide mechanistic insight into�the role of specific epigenetic marks, both as indicators of current metabolic status and as predictors of the future risk of obesity and metabolic disease. A particularly important aspect of animal studies is that they allow for the assessment of epigenetic changes within target tissues, including the liver and hypothalamus, which is much more difficult in humans. Moreover, the ability to harvest large quantities of fresh tissue makes it possible to assess multiple chromatin marks as well as DNA methylation. Some of these epigenetic modifications either alone or in combination may be responsive to environmental programming. In animal models, it is also possible to study multiple generations of offspring and thus enable differentiation between trans-generational and intergenerational transmission of obesity risk mediated by epigenetic memory of parental nutritional status, which cannot be easily distinguished in human studies. We use the former term for meiotic transmission of risk in the absence of continued exposure while the latter primarily entails direct transmission of risk through metabolic reprogramming of the fetus or gametes.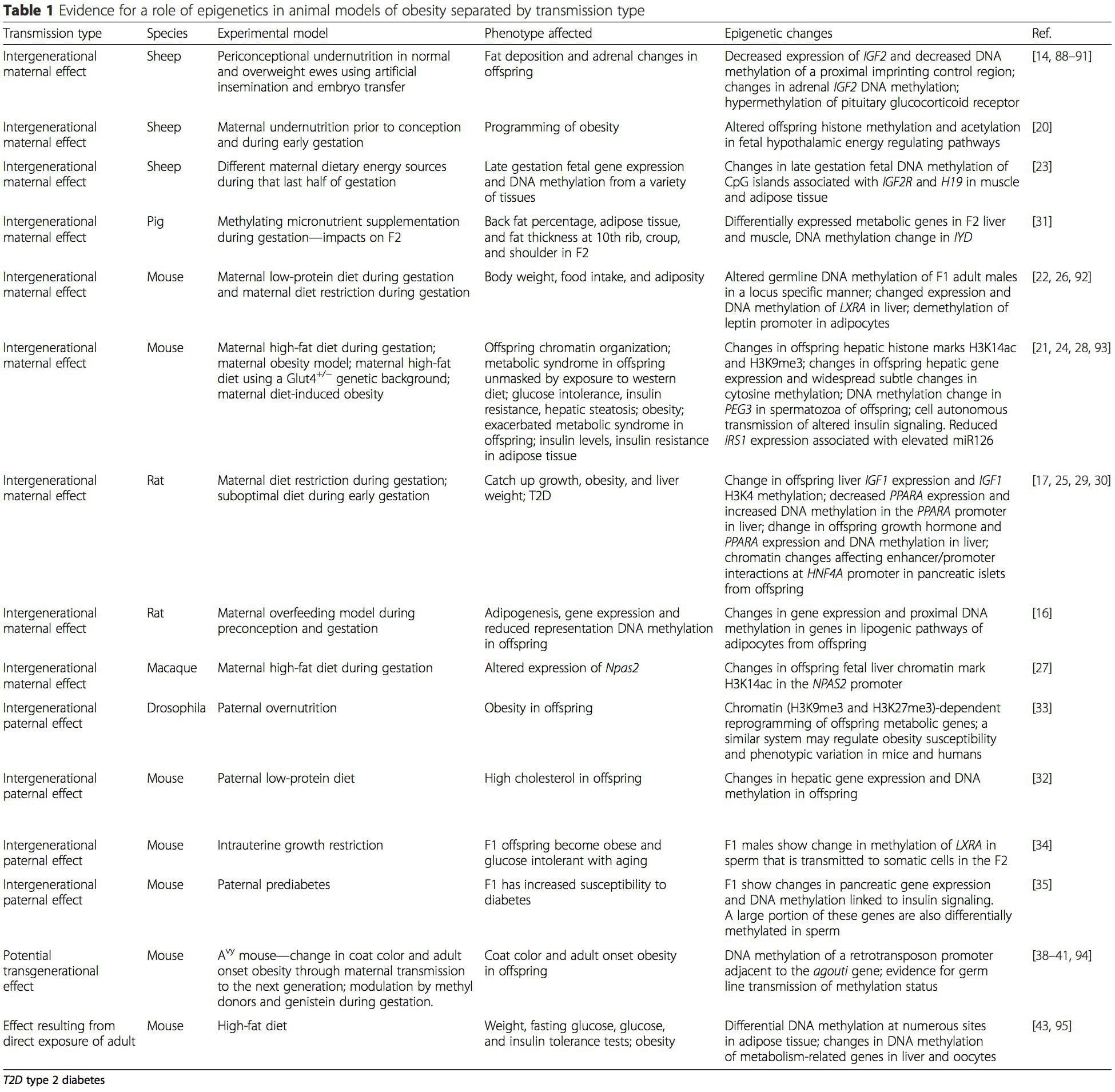 (i) Epigenetic Changes In Offspring Associated With Maternal Nutrition During Gestation
(i) Epigenetic Changes In Offspring Associated With Maternal Nutrition During Gestation Maternal nutritional supplementation, undernutrition, and over nutrition during pregnancy can alter fat deposition and energy homeostasis in offspring [11, 13�15, 19]. Associated with these effects in the offspring are changes in DNA methylation, histone post-translational modifications, and gene expression for several target genes,�especially genes regulating fatty acid metabolism and insulin signaling [16, 17, 20�30]. The diversity of animal models used in these studies and the common metabolic pathways impacted suggest an evolutionarily conserved adaptive response mediated by epigenetic modification. However, few of the specific identified genes and epigenetic changes have been cross-validated in related studies, and large-scale genome-wide investigations have typically not been applied. A major hindrance to comparison of these studies is the different develop mental windows subjected to nutritional challenge, which may cause considerably different outcomes. Proof that the epigenetic changes are causal rather than being associated with offspring phenotypic changes is also required. This will necessitate the identification of a parental nutritionally induced epigenetic �memory� response that precedes development of the altered phenotype in offspring.
Maternal nutritional supplementation, undernutrition, and over nutrition during pregnancy can alter fat deposition and energy homeostasis in offspring [11, 13�15, 19]. Associated with these effects in the offspring are changes in DNA methylation, histone post-translational modifications, and gene expression for several target genes,�especially genes regulating fatty acid metabolism and insulin signaling [16, 17, 20�30]. The diversity of animal models used in these studies and the common metabolic pathways impacted suggest an evolutionarily conserved adaptive response mediated by epigenetic modification. However, few of the specific identified genes and epigenetic changes have been cross-validated in related studies, and large-scale genome-wide investigations have typically not been applied. A major hindrance to comparison of these studies is the different develop mental windows subjected to nutritional challenge, which may cause considerably different outcomes. Proof that the epigenetic changes are causal rather than being associated with offspring phenotypic changes is also required. This will necessitate the identification of a parental nutritionally induced epigenetic �memory� response that precedes development of the altered phenotype in offspring. Emerging studies have demonstrated that paternal plane of nutrition can impact offspring fat deposition and epigenetic marks [31�34]. One recent investigation using mice has demonstrated that paternal pre-diabetes leads to increased susceptibility to diabetes in F1 offspring with associated changes in pancreatic gene expression and DNA methylation linked to insulin signaling [35]. Importantly, there was an overlap of these epigenetic changes in pancreatic islets and sperm suggesting germ line inheritance. However, most of these studies, although intriguing in their implications, are limited in the genomic scale of investigation and frequently show weak and somewhat transient epigenetic alterations associated with mild metabolic phenotypes in offspring.
Emerging studies have demonstrated that paternal plane of nutrition can impact offspring fat deposition and epigenetic marks [31�34]. One recent investigation using mice has demonstrated that paternal pre-diabetes leads to increased susceptibility to diabetes in F1 offspring with associated changes in pancreatic gene expression and DNA methylation linked to insulin signaling [35]. Importantly, there was an overlap of these epigenetic changes in pancreatic islets and sperm suggesting germ line inheritance. However, most of these studies, although intriguing in their implications, are limited in the genomic scale of investigation and frequently show weak and somewhat transient epigenetic alterations associated with mild metabolic phenotypes in offspring.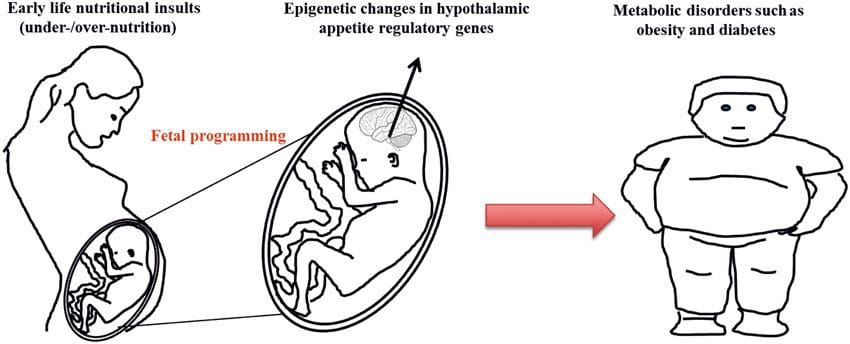 Stable transmission of epigenetic information across multiple generations is well described in plant systems and C. elegans, but its significance in mammals is still much debated [36, 37]. An epigenetic basis for grand- parental transmission of phenotypes in response to dietary exposures has been well established, including in livestock species [31]. The most influential studies demonstrating effects of epigenetic transmission impacting offspring phenotype have used the example of the viable yellow agouti (Avy) mouse [38]. In this mouse, an insertion of a retrotransposon upstream of the agouti gene causes its constitutive expression and consequent yellow coat color and adult onset obesity. Maternal transmission through the germ line results in DNA methylation�mediated silencing of agouti expression resulting in wild-type coat color and lean phenotype of the offspring [39, 40]. Importantly, subsequent studies in these mice demonstrated that maternal exposure to methyl donors causes a shift in coat color [41]. One study has reported transmission of a phenotype to the F3 generation and alterations in expression of large number of genes in response to protein restriction in F0 [42]; however, alterations in expression were highly variable and a direct link to epigenetic changes was not identified in this system.
Stable transmission of epigenetic information across multiple generations is well described in plant systems and C. elegans, but its significance in mammals is still much debated [36, 37]. An epigenetic basis for grand- parental transmission of phenotypes in response to dietary exposures has been well established, including in livestock species [31]. The most influential studies demonstrating effects of epigenetic transmission impacting offspring phenotype have used the example of the viable yellow agouti (Avy) mouse [38]. In this mouse, an insertion of a retrotransposon upstream of the agouti gene causes its constitutive expression and consequent yellow coat color and adult onset obesity. Maternal transmission through the germ line results in DNA methylation�mediated silencing of agouti expression resulting in wild-type coat color and lean phenotype of the offspring [39, 40]. Importantly, subsequent studies in these mice demonstrated that maternal exposure to methyl donors causes a shift in coat color [41]. One study has reported transmission of a phenotype to the F3 generation and alterations in expression of large number of genes in response to protein restriction in F0 [42]; however, alterations in expression were highly variable and a direct link to epigenetic changes was not identified in this system. While many studies have identified diet-associated epigenetic changes in animal models using candidate site-specific regions, there have been few genome-wide analyses undertaken. A recent study focussed on determining the direct epigenetic impact of high-fat diets/ diet-induced obesity in adult mice using genome-wide gene expression and DNA methylation analyses [43]. This study identified 232 differentially methylated regions (DMRs) in adipocytes from control and high-fat fed mice. Importantly, the corresponding human regions for the murine DMRs were also differentially methylated in adipose tissue from a population of obese and lean humans, thereby highlighting the remarkable evolutionary conservation of these regions. This result emphasizes the likely importance of the identified DMRs in regulating energy homeostasis in mammals.
While many studies have identified diet-associated epigenetic changes in animal models using candidate site-specific regions, there have been few genome-wide analyses undertaken. A recent study focussed on determining the direct epigenetic impact of high-fat diets/ diet-induced obesity in adult mice using genome-wide gene expression and DNA methylation analyses [43]. This study identified 232 differentially methylated regions (DMRs) in adipocytes from control and high-fat fed mice. Importantly, the corresponding human regions for the murine DMRs were also differentially methylated in adipose tissue from a population of obese and lean humans, thereby highlighting the remarkable evolutionary conservation of these regions. This result emphasizes the likely importance of the identified DMRs in regulating energy homeostasis in mammals.
 (i) Genetic association studies. Genetic polymorphisms that are associated with an increased risk of developing particular conditions are a priori linked to the causative genes. The presence of differential�methylation in such regions infers functional relevance of these epigenetic changes in controlling expression of the proximal gene(s). There are strong cis-acting genetic effects underpinning much epigenetic variation [7, 45], and in population-based studies, methods that use genetic surrogates to infer a causal or mediating role of epigenome differences have been applied [7, 46�48]. The use of familial genetic information can also lead to the identification of potentially causative candidate regions showing phenotype-related differential methylation [49].
(i) Genetic association studies. Genetic polymorphisms that are associated with an increased risk of developing particular conditions are a priori linked to the causative genes. The presence of differential�methylation in such regions infers functional relevance of these epigenetic changes in controlling expression of the proximal gene(s). There are strong cis-acting genetic effects underpinning much epigenetic variation [7, 45], and in population-based studies, methods that use genetic surrogates to infer a causal or mediating role of epigenome differences have been applied [7, 46�48]. The use of familial genetic information can also lead to the identification of potentially causative candidate regions showing phenotype-related differential methylation [49].
 From these studies, altered methylation of PGC1A, HIF3A, ABCG1, and CPT1A and the previously described RXRA [18] have emerged as biomarkers associated with, or perhaps predictive of, metabolic health that are also plausible candidates for a role in development of metabolic disease.
From these studies, altered methylation of PGC1A, HIF3A, ABCG1, and CPT1A and the previously described RXRA [18] have emerged as biomarkers associated with, or perhaps predictive of, metabolic health that are also plausible candidates for a role in development of metabolic disease.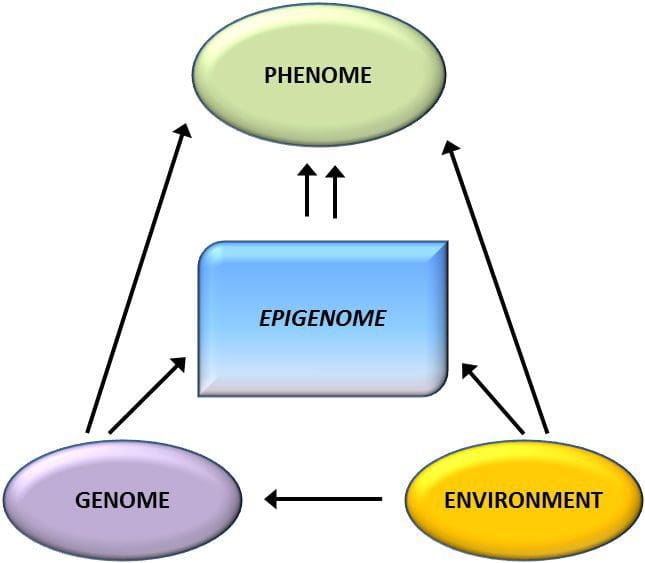 Epigenetic variation is highly influenced by the underlying genetic variation, with genotype estimated to explain ~20�40 % of the variation [6, 8]. Recently, a number of studies have begun to integrate methylome and genotype data to identify methylation quantitative trait loci (meQTL) associated with disease phenotypes. For instance, in adipose tissue, an meQTL overlapping�with a BMI genetic risk locus has been identified in an enhancer element upstream of ADCY3 [8]. Other studies have also identified overlaps between known obesity and T2DM risk loci and DMRs associated with obesity and T2DM [43, 48, 62]. Methylation of a number of such DMRs was also modulated by high-fat feeding in mice [43] and weight loss in humans [64]. These results identify an intriguing link between genetic variations linked with disease susceptibility and their association with regions of the genome that undergo epigenetic modifications in response to nutritional challenges, implying a causal relationship. The close connection between genetic and epigenetic variation may signify their essential roles in generating individual variation [65, 66]. However, while these findings suggest that DNA methylation may be a mediator of genetic effects, it is also important to consider that both genetic and epigenetic processes could act independently on the same genes. Twin studies [8, 63, 67] can provide important insights and indicate that inter-individual differences in levels of DNA methylation arise predominantly from non-shared environment and stochastic influences, minimally from shared environmental effects, but also with a significant impact of genetic variation.
Epigenetic variation is highly influenced by the underlying genetic variation, with genotype estimated to explain ~20�40 % of the variation [6, 8]. Recently, a number of studies have begun to integrate methylome and genotype data to identify methylation quantitative trait loci (meQTL) associated with disease phenotypes. For instance, in adipose tissue, an meQTL overlapping�with a BMI genetic risk locus has been identified in an enhancer element upstream of ADCY3 [8]. Other studies have also identified overlaps between known obesity and T2DM risk loci and DMRs associated with obesity and T2DM [43, 48, 62]. Methylation of a number of such DMRs was also modulated by high-fat feeding in mice [43] and weight loss in humans [64]. These results identify an intriguing link between genetic variations linked with disease susceptibility and their association with regions of the genome that undergo epigenetic modifications in response to nutritional challenges, implying a causal relationship. The close connection between genetic and epigenetic variation may signify their essential roles in generating individual variation [65, 66]. However, while these findings suggest that DNA methylation may be a mediator of genetic effects, it is also important to consider that both genetic and epigenetic processes could act independently on the same genes. Twin studies [8, 63, 67] can provide important insights and indicate that inter-individual differences in levels of DNA methylation arise predominantly from non-shared environment and stochastic influences, minimally from shared environmental effects, but also with a significant impact of genetic variation. Prenatal environment: Two recently published studies made use of human populations that experienced �natural� variations in nutrient supply to study the impact of maternal nutrition before or during pregnancy on DNA methylation in the offspring [68, 69]. The first study used a Gambian mother-child cohort to show that both seasonal variations in maternal methyl donor intake during pregnancy and maternal pre-pregnancy BMI were associated with altered methylation in the infants [69]. The second study utilized adult offspring from the Dutch Hunger Winter cohort to investigate the effect of prenatal exposure to an acute period of severe maternal undernutrition on DNA methylation of genes involved in growth and metabolism in adulthood [68]. The results highlighted the importance of the timing of the exposure in its impact on the epigenome, since significant epigenetic effects were only identified in individuals exposed to famine during early gestation. Importantly, the epigenetic changes occurred in conjunction with increased BMI; however, it was not possible to establish in this study whether these changes were present earlier in life or a consequence of the higher BMI.
Prenatal environment: Two recently published studies made use of human populations that experienced �natural� variations in nutrient supply to study the impact of maternal nutrition before or during pregnancy on DNA methylation in the offspring [68, 69]. The first study used a Gambian mother-child cohort to show that both seasonal variations in maternal methyl donor intake during pregnancy and maternal pre-pregnancy BMI were associated with altered methylation in the infants [69]. The second study utilized adult offspring from the Dutch Hunger Winter cohort to investigate the effect of prenatal exposure to an acute period of severe maternal undernutrition on DNA methylation of genes involved in growth and metabolism in adulthood [68]. The results highlighted the importance of the timing of the exposure in its impact on the epigenome, since significant epigenetic effects were only identified in individuals exposed to famine during early gestation. Importantly, the epigenetic changes occurred in conjunction with increased BMI; however, it was not possible to establish in this study whether these changes were present earlier in life or a consequence of the higher BMI. Postnatal environment: The epigenome is established de novo during embryonic development, and therefore, the prenatal environment most likely has the most significant impact on the epigenome. However, it is now clear that changes do occur in the �mature� epigenome under the influence of a range of conditions, including aging, exposure to toxins, and dietary alterations. For example, changes in DNA methylation in numerous genes in skeletal muscle and PGC1A in adipose tissue have been demonstrated in response to a high-fat diet [75, 76]. Interventions to lose body fat mass have also been associated with changes in DNA methylation. Studies have reported that the DNA methylation profiles of adipose tissue [43, 64], peripheral blood mononuclear cells [77], and muscle tissue [78] in formerly obese patients become more similar to the profiles of lean subjects following weight loss. Weight loss surgery also partially reversed non-alcoholic fatty liver disease-associated methylation changes in liver [79] and in another study led to hypomethylation of multiple obesity candidate genes, with more pronounced effects in subcutaneous compared to omental (visceral) fat [64]. Accumulating evidence suggests that exercise interventions can also influence DNA methylation. Most of these studies have been conducted in lean individuals [80�82], but one exercise study in obese T2DM subjects also demonstrated changes in DNA methylation, including in genes involved in fatty acid and glucose transport [83]. Epigenetic changes also occur with aging, and recent data suggest a role of obesity in augmenting them [9, 84, 85]. Obesity accelerated the epigenetic age of liver tissue, but in contrast to the findings described above, this effect was not reversible after weight loss [84].
Postnatal environment: The epigenome is established de novo during embryonic development, and therefore, the prenatal environment most likely has the most significant impact on the epigenome. However, it is now clear that changes do occur in the �mature� epigenome under the influence of a range of conditions, including aging, exposure to toxins, and dietary alterations. For example, changes in DNA methylation in numerous genes in skeletal muscle and PGC1A in adipose tissue have been demonstrated in response to a high-fat diet [75, 76]. Interventions to lose body fat mass have also been associated with changes in DNA methylation. Studies have reported that the DNA methylation profiles of adipose tissue [43, 64], peripheral blood mononuclear cells [77], and muscle tissue [78] in formerly obese patients become more similar to the profiles of lean subjects following weight loss. Weight loss surgery also partially reversed non-alcoholic fatty liver disease-associated methylation changes in liver [79] and in another study led to hypomethylation of multiple obesity candidate genes, with more pronounced effects in subcutaneous compared to omental (visceral) fat [64]. Accumulating evidence suggests that exercise interventions can also influence DNA methylation. Most of these studies have been conducted in lean individuals [80�82], but one exercise study in obese T2DM subjects also demonstrated changes in DNA methylation, including in genes involved in fatty acid and glucose transport [83]. Epigenetic changes also occur with aging, and recent data suggest a role of obesity in augmenting them [9, 84, 85]. Obesity accelerated the epigenetic age of liver tissue, but in contrast to the findings described above, this effect was not reversible after weight loss [84].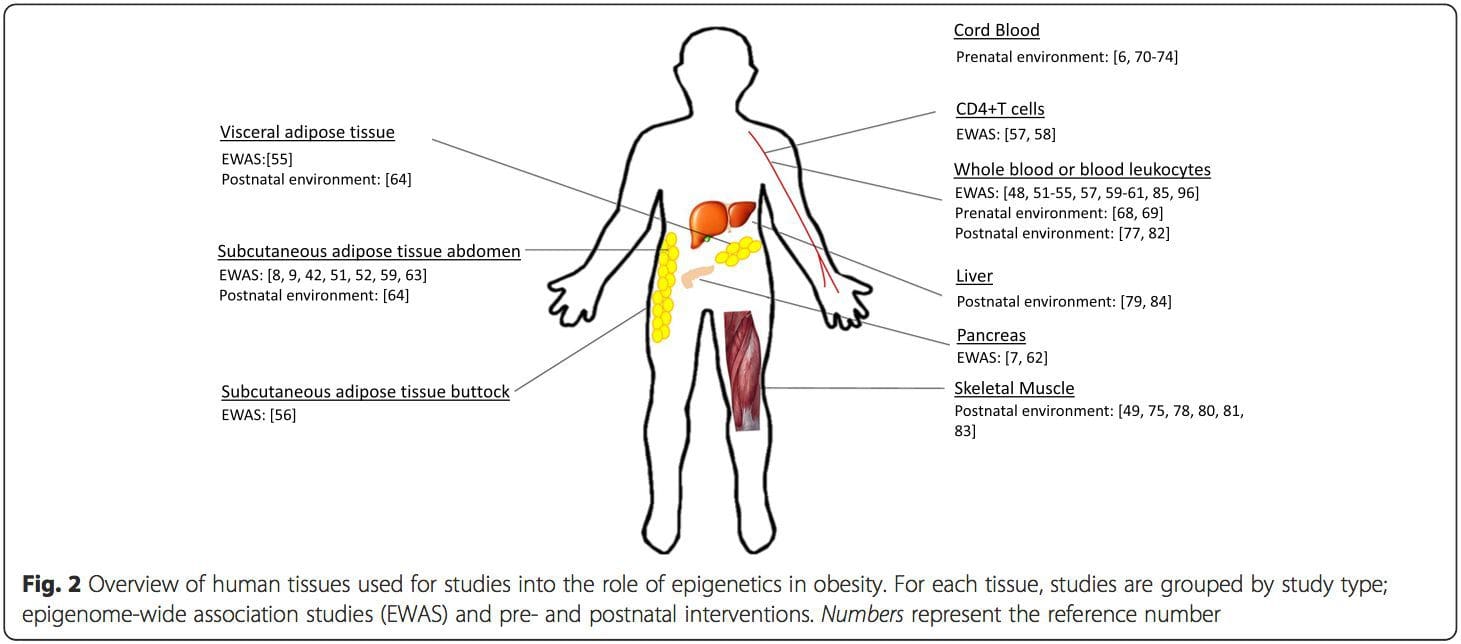 Conclusions
Conclusions
 Chronic undernutrition is characterized by a progressive reduction of the�fat-free mass (FFM) and fat mass (FM)�and �which has deleterious consequences on health. Undernutrition is insufficiently screened and treated in hospitalized or at-risk patients despite its high prevalence and negative impact on mortality, morbidity, length of stay (LOS), quality of life, and costs [1�4]. The risk of underestimating hospital undernutrition is likely to worsen in the next decades because of the increasing prevalence of overweight, obesity, and chronic diseases and the increased number of elderly subjects. These clinical conditions are associated with FFM loss (sarcopenia). Therefore, an increased number of patients with FFM loss and sarcopenic obesity will be seen in the future.
Chronic undernutrition is characterized by a progressive reduction of the�fat-free mass (FFM) and fat mass (FM)�and �which has deleterious consequences on health. Undernutrition is insufficiently screened and treated in hospitalized or at-risk patients despite its high prevalence and negative impact on mortality, morbidity, length of stay (LOS), quality of life, and costs [1�4]. The risk of underestimating hospital undernutrition is likely to worsen in the next decades because of the increasing prevalence of overweight, obesity, and chronic diseases and the increased number of elderly subjects. These clinical conditions are associated with FFM loss (sarcopenia). Therefore, an increased number of patients with FFM loss and sarcopenic obesity will be seen in the future.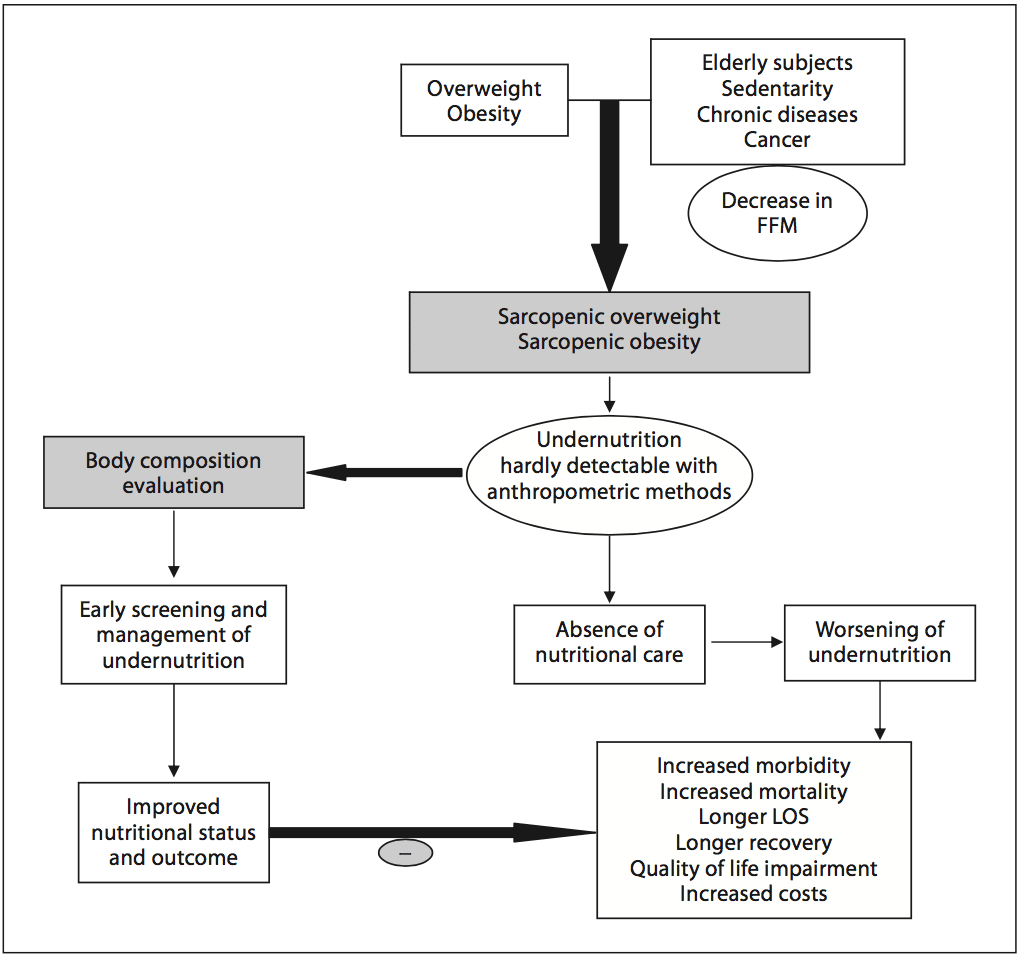
 Academic societies encourage systematic screening of undernutrition at hospital admission and during the hospital stay [14]. The detection of undernutrition is generally based on measurements of weight and height, calculations of BMI, and the percentage of weight loss. Nevertheless, screening of undernutrition is infrequent in hospitalized or nutritionally at-risk ambulatory patients. For example, in France, surveys performed by the French Health Authority [15] indicate that: (i) weight alone, (ii) weight with BMI or percentage of weight loss, and (iii) weight, BMI,�and percentage of weight loss are reported in only 55, 30, and 8% of the hospitalized patients� records, respectively. Several issues, which could be improved by specific educational programs, explain the lack of implementation of nutritional screening in hospitals (table 1). In addition, the accuracy of the clinical screening of undernutrition could be limited at hospital admission. Indeed, patients with undernutrition may have the same BMI as sex- and age- matched healthy controls but a significantly decreased FFM hidden by an expansion of the FM and the total body water which can be measured by bioelectrical impedance analysis (BIA) [13]. This example illustrates that body composition evaluation allows a more accurate identification of FFM loss than body weight loss or BMI decrease. The lack of sensitivity and specificity of weight, BMI, and percentage of weight loss argue for the need for other methods to evaluate the nutritional status.
Academic societies encourage systematic screening of undernutrition at hospital admission and during the hospital stay [14]. The detection of undernutrition is generally based on measurements of weight and height, calculations of BMI, and the percentage of weight loss. Nevertheless, screening of undernutrition is infrequent in hospitalized or nutritionally at-risk ambulatory patients. For example, in France, surveys performed by the French Health Authority [15] indicate that: (i) weight alone, (ii) weight with BMI or percentage of weight loss, and (iii) weight, BMI,�and percentage of weight loss are reported in only 55, 30, and 8% of the hospitalized patients� records, respectively. Several issues, which could be improved by specific educational programs, explain the lack of implementation of nutritional screening in hospitals (table 1). In addition, the accuracy of the clinical screening of undernutrition could be limited at hospital admission. Indeed, patients with undernutrition may have the same BMI as sex- and age- matched healthy controls but a significantly decreased FFM hidden by an expansion of the FM and the total body water which can be measured by bioelectrical impedance analysis (BIA) [13]. This example illustrates that body composition evaluation allows a more accurate identification of FFM loss than body weight loss or BMI decrease. The lack of sensitivity and specificity of weight, BMI, and percentage of weight loss argue for the need for other methods to evaluate the nutritional status. In 2008, twelve and thirty percent of the worldwide adult population was obese or overweight; this is two times higher than in 1980 [16]. The prevalence of overweight and obesity is also increasing in hospitalized patients. A 10-year comparative survey performed in a European hospital showed an increase in patients� BMI, together with a shorter LOS [17]. The BMI increase masks undernutrition and FFM loss at hospital admission. The increased prevalence of obesity in an aging population has led to the recognition of a new nutritional entity: �sarcopenic obesity� [18]. Sarcopenic obesity is characterized by increased FM and reduced FFM with a normal or high body weight. The emergence of the concept of sarcopenic obesity will increase the number of situations associated with a lack of sensitivity of the calculations of BMI and�body weight change for the early detection of FFM loss. This supports a larger use of body composition evaluation for the assessment and follow-up of nutritional status in clinical practice (fig. 1).
In 2008, twelve and thirty percent of the worldwide adult population was obese or overweight; this is two times higher than in 1980 [16]. The prevalence of overweight and obesity is also increasing in hospitalized patients. A 10-year comparative survey performed in a European hospital showed an increase in patients� BMI, together with a shorter LOS [17]. The BMI increase masks undernutrition and FFM loss at hospital admission. The increased prevalence of obesity in an aging population has led to the recognition of a new nutritional entity: �sarcopenic obesity� [18]. Sarcopenic obesity is characterized by increased FM and reduced FFM with a normal or high body weight. The emergence of the concept of sarcopenic obesity will increase the number of situations associated with a lack of sensitivity of the calculations of BMI and�body weight change for the early detection of FFM loss. This supports a larger use of body composition evaluation for the assessment and follow-up of nutritional status in clinical practice (fig. 1).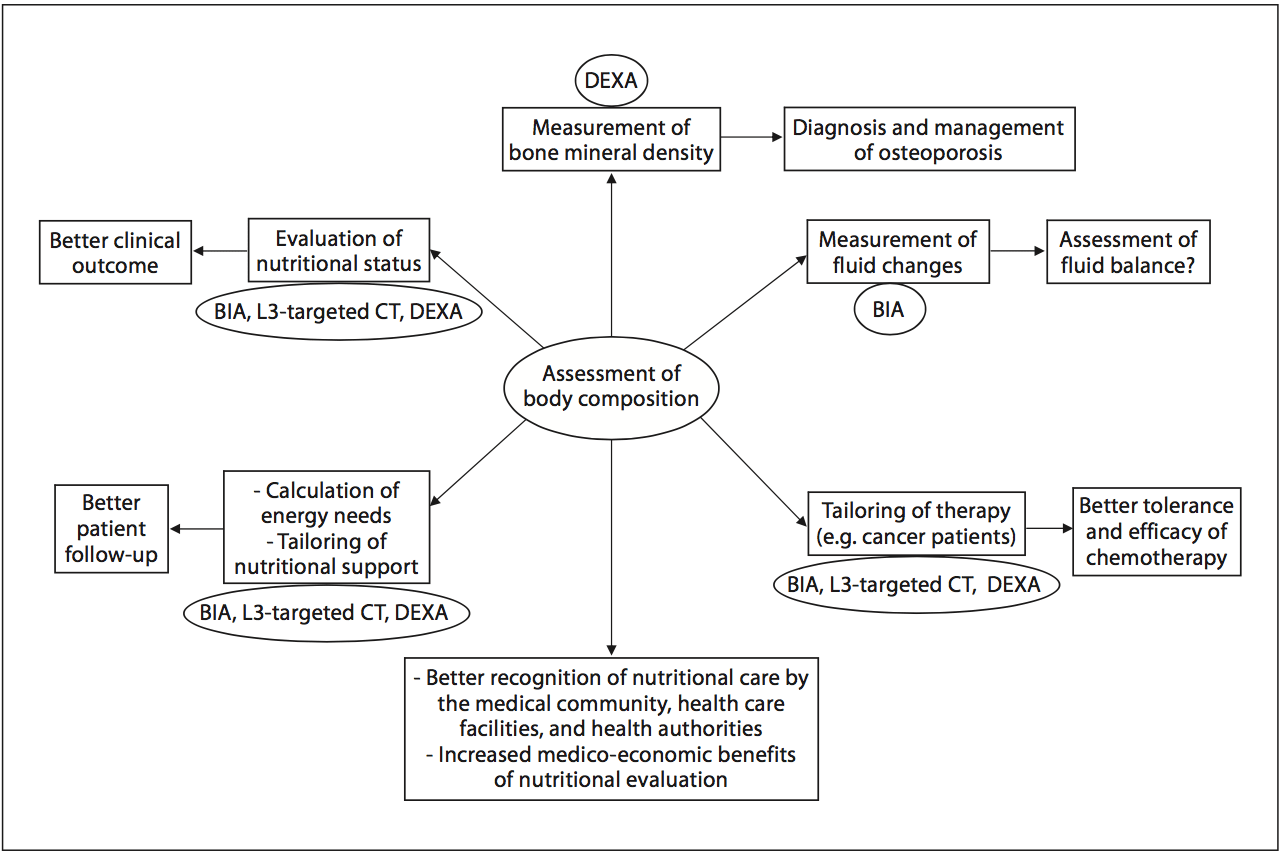 Fig. 2. Current and potential applications of body composition evaluation in clinical practice. The applications are indicated in the boxes, and the body composition methods that could be used for each application are indicated inside the circles. The most used application of body composition evaluation is the measurement of bone mineral density by DEXA for the diagnosis and management of osteoporosis. Although a low FFM is associated with worse clinical outcomes, FFM evaluation is not yet implemented enough in clinical practice. However, by allowing early detection of undernutrition, body composition evaluation could improve the clinical outcome. Body composition evaluation could also be used to follow up nutritional status, calculate energy needs, tailor nutritional support, and assess fluid changes during perioperative period and renal insufficiency. Recent evidence indicates that�a low FFM is associated with a higher toxicity of some chemo- therapy drugs in cancer patients. Thus, by allowing tailoring of the chemotherapy doses to the FFM in cancer patients, body com- position evaluation should improve the tolerance and the efficacy of chemotherapy. BIA, L3-targeted CT, and DEXA could be used for the assessment of nutritional status, the calculation of energy needs, and the tailoring of nutritional support and therapy. Further studies are warranted to validate BIA as an accurate method for fluid balance measurement. By integrating body composition evaluation into the management of different clinical conditions, all of these potential applications would lead to a better recognition of nutritional care by the medical community, the health care facilities, and the health authorities, as well as to an increase in the medico-economic benefits of the nutritional evaluation.
Fig. 2. Current and potential applications of body composition evaluation in clinical practice. The applications are indicated in the boxes, and the body composition methods that could be used for each application are indicated inside the circles. The most used application of body composition evaluation is the measurement of bone mineral density by DEXA for the diagnosis and management of osteoporosis. Although a low FFM is associated with worse clinical outcomes, FFM evaluation is not yet implemented enough in clinical practice. However, by allowing early detection of undernutrition, body composition evaluation could improve the clinical outcome. Body composition evaluation could also be used to follow up nutritional status, calculate energy needs, tailor nutritional support, and assess fluid changes during perioperative period and renal insufficiency. Recent evidence indicates that�a low FFM is associated with a higher toxicity of some chemo- therapy drugs in cancer patients. Thus, by allowing tailoring of the chemotherapy doses to the FFM in cancer patients, body com- position evaluation should improve the tolerance and the efficacy of chemotherapy. BIA, L3-targeted CT, and DEXA could be used for the assessment of nutritional status, the calculation of energy needs, and the tailoring of nutritional support and therapy. Further studies are warranted to validate BIA as an accurate method for fluid balance measurement. By integrating body composition evaluation into the management of different clinical conditions, all of these potential applications would lead to a better recognition of nutritional care by the medical community, the health care facilities, and the health authorities, as well as to an increase in the medico-economic benefits of the nutritional evaluation.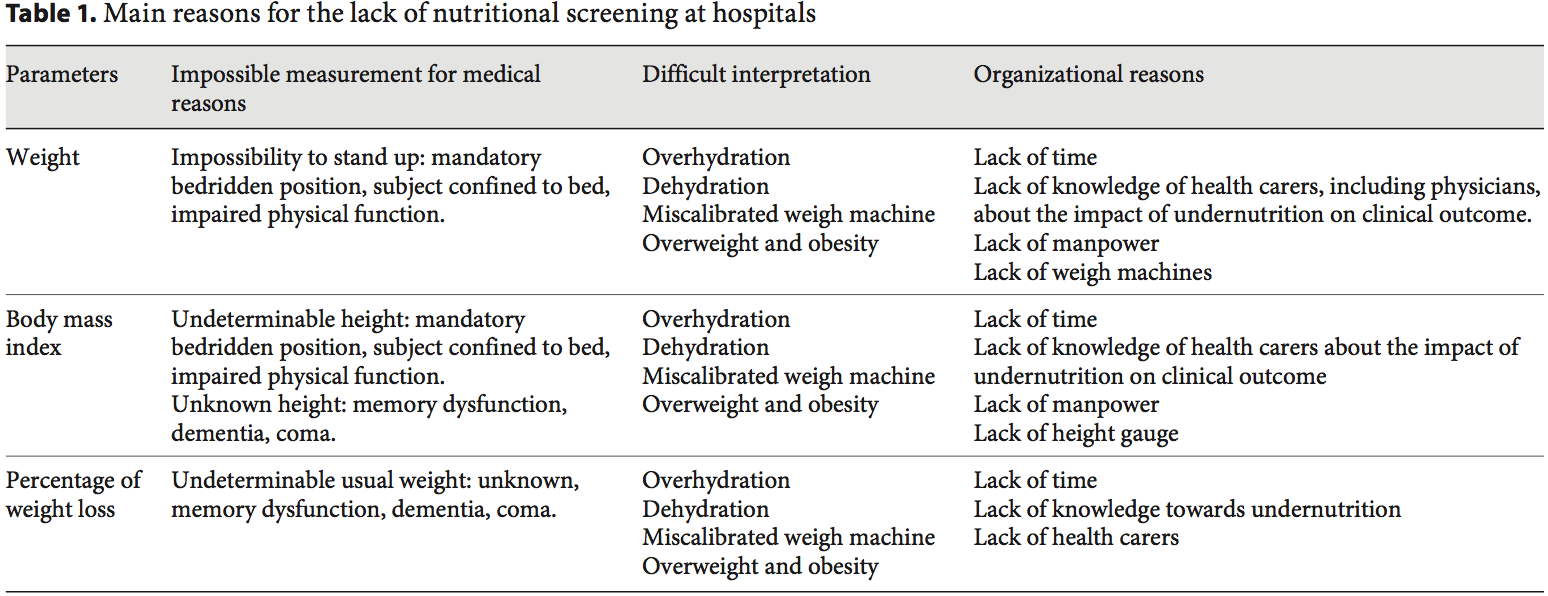
 Body Composition Techniques For FFM Measurement
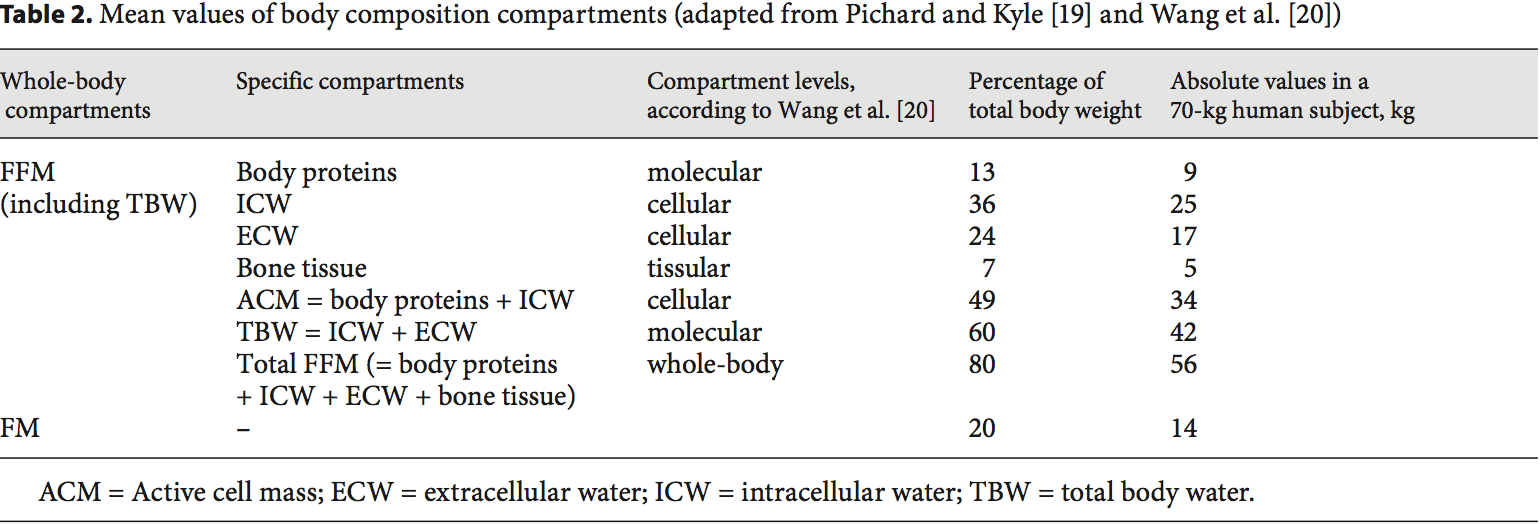
Body Composition Techniques For FFM Measurement Body composition evaluation allows measurement of the major body compartments: FFM (including bone mineral tissue), FM, and total body water. Table 2 shows indicative values of the body composition of a healthy subject weighing 70 kg. In several clinical situations, i.e. hospital admission, chronic obstructive pulmonary dis- ease (COPD) [21�23], dialysis [24�26], chronic heart failure [27], amyotrophic lateral sclerosis [28], cancer [5, 29], liver transplantation [30], nursing home residence [31], and Alzheimer�s disease [32], changes in body compartments are detected with the techniques of body composition evaluation. At hospital admission, body composition evaluation could be used for the detection of FFM loss and undernutrition. Indeed, FFM and the FFM index (FFMI) [FFM (kg)/height (m2)] measured by BIA are significantly lower in hospitalized patients (n = 995) than in age-, height-, and sex-matched controls (n = 995) [3]. Conversely, clinical tools of nutritional status assessment, such as BMI, subjective global assessment, or mini-nutritional assessment, are not accurate enough to estimate FFM loss and nutritional status [30, 32�34]. In 441 patients with non-small cell lung cancer, FFM loss deter- mined by computerized tomography (CT) was observed in each BMI category [7], and in young adults with all�types of cancer, an increase in FM together with a de- crease in FFM were reported [29]. These findings reveal the lack of sensitivity of BMI to detect FFM loss. More- over, the FFMI is a more sensitive determinant of LOS than a weight loss over 10% or a BMI below 20 [3]. In COPD, the assessment of FFM by BIA is a more sensitive method to detect undernutrition than anthropometry [33, 35]. BIA is also more accurate at assessing nutrition- al status in children with severe neurologic impairment than the measurement of skin fold thickness [36].
Body composition evaluation allows measurement of the major body compartments: FFM (including bone mineral tissue), FM, and total body water. Table 2 shows indicative values of the body composition of a healthy subject weighing 70 kg. In several clinical situations, i.e. hospital admission, chronic obstructive pulmonary dis- ease (COPD) [21�23], dialysis [24�26], chronic heart failure [27], amyotrophic lateral sclerosis [28], cancer [5, 29], liver transplantation [30], nursing home residence [31], and Alzheimer�s disease [32], changes in body compartments are detected with the techniques of body composition evaluation. At hospital admission, body composition evaluation could be used for the detection of FFM loss and undernutrition. Indeed, FFM and the FFM index (FFMI) [FFM (kg)/height (m2)] measured by BIA are significantly lower in hospitalized patients (n = 995) than in age-, height-, and sex-matched controls (n = 995) [3]. Conversely, clinical tools of nutritional status assessment, such as BMI, subjective global assessment, or mini-nutritional assessment, are not accurate enough to estimate FFM loss and nutritional status [30, 32�34]. In 441 patients with non-small cell lung cancer, FFM loss deter- mined by computerized tomography (CT) was observed in each BMI category [7], and in young adults with all�types of cancer, an increase in FM together with a de- crease in FFM were reported [29]. These findings reveal the lack of sensitivity of BMI to detect FFM loss. More- over, the FFMI is a more sensitive determinant of LOS than a weight loss over 10% or a BMI below 20 [3]. In COPD, the assessment of FFM by BIA is a more sensitive method to detect undernutrition than anthropometry [33, 35]. BIA is also more accurate at assessing nutrition- al status in children with severe neurologic impairment than the measurement of skin fold thickness [36]. FFM loss is correlated with survival in different clinical settings [5, 21�28, 37]. In patients with amyotrophic lateral sclerosis, an FM increase, but not an FFM in- crease, measured by BIA, was correlated with survival during the course of the disease [28]. The relation between body composition and mortality has not yet been demonstrated in the intensive care unit. The relation between body composition and mortality has been demonstrated with anthropometric methods, BIA, and CT. Measurement of the mid-arm muscle circumference is an easy tool to diagnose sarcopenia [38]. The mid-arm muscle circumference has been shown to be correlated with survival in patients with cirrhosis [39, 40], HIV infection [41], and COPD in a stronger way than BMI [42]. The relation between FFM loss and mortality has been extensively shown with BIA [21�28, 31, 37], which is the most used method. Recently, very interesting data suggest that CT could evaluate the disease prognosis in relation to muscle wasting. In obese cancer patients, sarcopenia as assessed by CT measurement of the total skeletal muscle cross-sectional area is an independent predictor of the survival of patients with bronchopulmonary [5, 7], gastrointestinal [5], and pancreatic cancers [6]. FFM assessed by measurement of the mid-thigh muscle cross- sectional area by CT is also predictive of mortality in COPD patients with severe chronic respiratory insufficiency [43]. In addition to mortality, a low FFMI at hospital admission is significantly associated with an in- creased LOS [3, 44]. A bicentric controlled population study performed in 1,717 hospitalized patients indicates that both loss of FFM and excess of FM negatively affect the LOS [44]. Patients with sarcopenic obesity are most at risk of increased LOS. This study also found that ex- cess FM reduces the sensitivity of BMI to detect nutritional depletion [44]. Together with the observation that the BMI of hospitalized patients has increased during the last decade [17], these findings suggest that FFM and�FFMI measurement should be used to evaluate nutritional status in hospitalized patients.
FFM loss is correlated with survival in different clinical settings [5, 21�28, 37]. In patients with amyotrophic lateral sclerosis, an FM increase, but not an FFM in- crease, measured by BIA, was correlated with survival during the course of the disease [28]. The relation between body composition and mortality has not yet been demonstrated in the intensive care unit. The relation between body composition and mortality has been demonstrated with anthropometric methods, BIA, and CT. Measurement of the mid-arm muscle circumference is an easy tool to diagnose sarcopenia [38]. The mid-arm muscle circumference has been shown to be correlated with survival in patients with cirrhosis [39, 40], HIV infection [41], and COPD in a stronger way than BMI [42]. The relation between FFM loss and mortality has been extensively shown with BIA [21�28, 31, 37], which is the most used method. Recently, very interesting data suggest that CT could evaluate the disease prognosis in relation to muscle wasting. In obese cancer patients, sarcopenia as assessed by CT measurement of the total skeletal muscle cross-sectional area is an independent predictor of the survival of patients with bronchopulmonary [5, 7], gastrointestinal [5], and pancreatic cancers [6]. FFM assessed by measurement of the mid-thigh muscle cross- sectional area by CT is also predictive of mortality in COPD patients with severe chronic respiratory insufficiency [43]. In addition to mortality, a low FFMI at hospital admission is significantly associated with an in- creased LOS [3, 44]. A bicentric controlled population study performed in 1,717 hospitalized patients indicates that both loss of FFM and excess of FM negatively affect the LOS [44]. Patients with sarcopenic obesity are most at risk of increased LOS. This study also found that ex- cess FM reduces the sensitivity of BMI to detect nutritional depletion [44]. Together with the observation that the BMI of hospitalized patients has increased during the last decade [17], these findings suggest that FFM and�FFMI measurement should be used to evaluate nutritional status in hospitalized patients. Numerous methods of body composition evaluation have been developed: anthropometry, including the 4-skinfold method [58], hydrodensitometry [58], in vivo neutron activation analysis [59], anthropogammametry from total body potassium-40 [60], nuclear magnetic resonance [61], dual-energy X-ray absorptiometry (DEXA) [62, 63], BIA [45, 64�66], and more recently CT [7, 43, 67]. DEXA, BIA, and CT appear to be the most convenient methods for clinical practice (fig. 2), while the other methods are reserved for scientific use.
Numerous methods of body composition evaluation have been developed: anthropometry, including the 4-skinfold method [58], hydrodensitometry [58], in vivo neutron activation analysis [59], anthropogammametry from total body potassium-40 [60], nuclear magnetic resonance [61], dual-energy X-ray absorptiometry (DEXA) [62, 63], BIA [45, 64�66], and more recently CT [7, 43, 67]. DEXA, BIA, and CT appear to be the most convenient methods for clinical practice (fig. 2), while the other methods are reserved for scientific use. The evaluation of FFM could be used for the calculation of energy needs, thus allowing the optimization of nutritional intakes according to nutritional needs. This could be of great interest in specific situations, such as severe neurologic disability, overweight, and obesity. In 61 children with severe neurologic impairment and intellectual disability, an equation integrating body composition had good agreement with the doubly labeled water method. It gave a better estimation of energy expenditure than did the Schofield predictive equation [36]. However, in 9 anorexia nervosa patients with a mean BMI of 13.7, pre- diction formulas of resting energy expenditure including FFM did not allow accurate prediction of the resting energy expenditure measured by indirect calorimetry [76]. In overweight or obese patients, the muscle catabolism in response to inflammation was the same as that observed�in patients with normal BMI. Indeed, despite a higher BMI, the FFM of overweight or obese individuals is similar (or slightly increased) to that of patients with normal BMI. Thus, the use of actual weight for the assessment of the energy needs of obese patients would result in over- feeding and its related complications. Therefore, the ex- perts recommend the use of indirect calorimetry or calculation of the energy needs of overweight or obese patients as follows: 15 kcal/kg actual weight/day or 20�25 kcal/kg ideal weight/day [77, 78], although these predictive formulas could be inaccurate in some clinical conditions [79]. In a US prospective study conducted in 33 ICU medical and surgical ventilated ICU patients, daily measurement of the active cell mass (table 2) by BIA was used to assess the adequacy between energy/protein intakes and needs. In that study, nutritional support with 30 kcal/ kg actual body weight/day energy and 1.5 g/kg/day protein allowed stabilization of the active cell mass [75]. Thus, follow-up of FFM by BIA could help optimize nutritional intakes when indirect calorimetry cannot be performed.
The evaluation of FFM could be used for the calculation of energy needs, thus allowing the optimization of nutritional intakes according to nutritional needs. This could be of great interest in specific situations, such as severe neurologic disability, overweight, and obesity. In 61 children with severe neurologic impairment and intellectual disability, an equation integrating body composition had good agreement with the doubly labeled water method. It gave a better estimation of energy expenditure than did the Schofield predictive equation [36]. However, in 9 anorexia nervosa patients with a mean BMI of 13.7, pre- diction formulas of resting energy expenditure including FFM did not allow accurate prediction of the resting energy expenditure measured by indirect calorimetry [76]. In overweight or obese patients, the muscle catabolism in response to inflammation was the same as that observed�in patients with normal BMI. Indeed, despite a higher BMI, the FFM of overweight or obese individuals is similar (or slightly increased) to that of patients with normal BMI. Thus, the use of actual weight for the assessment of the energy needs of obese patients would result in over- feeding and its related complications. Therefore, the ex- perts recommend the use of indirect calorimetry or calculation of the energy needs of overweight or obese patients as follows: 15 kcal/kg actual weight/day or 20�25 kcal/kg ideal weight/day [77, 78], although these predictive formulas could be inaccurate in some clinical conditions [79]. In a US prospective study conducted in 33 ICU medical and surgical ventilated ICU patients, daily measurement of the active cell mass (table 2) by BIA was used to assess the adequacy between energy/protein intakes and needs. In that study, nutritional support with 30 kcal/ kg actual body weight/day energy and 1.5 g/kg/day protein allowed stabilization of the active cell mass [75]. Thus, follow-up of FFM by BIA could help optimize nutritional intakes when indirect calorimetry cannot be performed. Body composition evaluation allows a qualitative assessment of body weight variations. The evaluation of body composition may help to document the efficiency of nutritional support during a patient�s follow-up of numerous clinical conditions, such as surgery [59], anorexia nervosa [76, 80], hematopoietic stem cell transplantation [81], COPD [82], ICU [83], lung transplantation [84], ulcerative colitis [59], Crohn�s disease [85], cancer [86, 87], HIV/AIDS [88], and acute stroke in elderly patients [89]. Body composition evaluation could be used for the follow-up of healthy elderly subjects [90]. Body composition evaluation allows characterization of the increase in body mass in terms of FFM and FM [81, 91]. After hematopoietic stem cell transplantation, the increase in BMI is the result of the increase in FM, but not of the increase in FFM [81]. Also, during recovery after an acute illness, weight gain 6 months after ICU discharge could be mostly related to an increase in FM (+7 kg) while FFM only increased by 2 kg; DEXA and air displacement plethysmography were used to measure the FM and FFM [91]. These two examples suggest that body composition evaluation could be helpful to decide the modification and/or the renewal of nutritional support. By identifying the patients gaining weight but reporting no or insufficient FFM, body composition evaluation could contribute to influencing the medical decision of continuing nutrition- al support that would have been stopped in the absence of body composition evaluation.
Body composition evaluation allows a qualitative assessment of body weight variations. The evaluation of body composition may help to document the efficiency of nutritional support during a patient�s follow-up of numerous clinical conditions, such as surgery [59], anorexia nervosa [76, 80], hematopoietic stem cell transplantation [81], COPD [82], ICU [83], lung transplantation [84], ulcerative colitis [59], Crohn�s disease [85], cancer [86, 87], HIV/AIDS [88], and acute stroke in elderly patients [89]. Body composition evaluation could be used for the follow-up of healthy elderly subjects [90]. Body composition evaluation allows characterization of the increase in body mass in terms of FFM and FM [81, 91]. After hematopoietic stem cell transplantation, the increase in BMI is the result of the increase in FM, but not of the increase in FFM [81]. Also, during recovery after an acute illness, weight gain 6 months after ICU discharge could be mostly related to an increase in FM (+7 kg) while FFM only increased by 2 kg; DEXA and air displacement plethysmography were used to measure the FM and FFM [91]. These two examples suggest that body composition evaluation could be helpful to decide the modification and/or the renewal of nutritional support. By identifying the patients gaining weight but reporting no or insufficient FFM, body composition evaluation could contribute to influencing the medical decision of continuing nutrition- al support that would have been stopped in the absence of body composition evaluation. In clinical situations when weight and BMI do not reflect the FFM, the evaluation of body composition should be used to adapt drug doses to the FFM and/or FM absolute values in every patient. This point has been recently illustrated in oncology patients with sarcopenic obesity. FFM loss was determined by CT as described above. In cancer patients, some therapies could affect body com- position by inducing muscle wasting [92]. In patients with advanced renal cell carcinoma [92], sorafenib induces a significant 8% loss of skeletal muscular mass at 12 months. In turn, muscle wasting in patients with BMI less than 25 was significantly associated with sorafenib toxicity in patients with metastatic renal cancer [8]. In metastatic breast cancer patients receiving capecitabine treatment, and in patients with colorectal cancer receiving 5-fluorouracile, using the convention of dosing per unit of body surface area, FFM loss was the determinant of chemotherapy toxicity [9, 10] and time to tumor progression [10]. In colorectal cancer patients administered 5-fluoruracil, low FFM is a significant predictor of toxicity only in female patients [9]. The variation in toxicity between women and men may be partially explained by the fact that FFM was lower in females. Indeed, FFM rep- resents the distribution volume of most cytotoxic chemo- therapy drugs. In 2,115 cancer patients, the individual variations in FFM could change by up to three times the distribution volume of the chemotherapy drug per body area unit [5]. Thus, administering the same doses of chemotherapy drugs to a patient with a low FFM compared to a patient with a normal FFM would increase the risk of chemotherapy toxicity [5]. These data suggest that FFM loss could have a direct impact on the clinical outcome of cancer patients. Decreasing chemotherapy doses in case of FFM loss could contribute to improving cancer patients� prognosis through the improvement of the tolerance of chemotherapy. These findings justify the systematic evaluation of body composition in all cancer patients in order to detect FFM loss, tailor chemotherapy doses according to FFM values, and then improve the efficacy- tolerance and cost-efficiency ratios of the therapeutic strategies [93]. Body composition evaluation should also be used to tailor the doses of drugs which are calculated based on patients� weight, e.g. corticosteroids, immuno-suppressors (infliximab, azathioprine or methotrexate), or sedatives (propofol).
In clinical situations when weight and BMI do not reflect the FFM, the evaluation of body composition should be used to adapt drug doses to the FFM and/or FM absolute values in every patient. This point has been recently illustrated in oncology patients with sarcopenic obesity. FFM loss was determined by CT as described above. In cancer patients, some therapies could affect body com- position by inducing muscle wasting [92]. In patients with advanced renal cell carcinoma [92], sorafenib induces a significant 8% loss of skeletal muscular mass at 12 months. In turn, muscle wasting in patients with BMI less than 25 was significantly associated with sorafenib toxicity in patients with metastatic renal cancer [8]. In metastatic breast cancer patients receiving capecitabine treatment, and in patients with colorectal cancer receiving 5-fluorouracile, using the convention of dosing per unit of body surface area, FFM loss was the determinant of chemotherapy toxicity [9, 10] and time to tumor progression [10]. In colorectal cancer patients administered 5-fluoruracil, low FFM is a significant predictor of toxicity only in female patients [9]. The variation in toxicity between women and men may be partially explained by the fact that FFM was lower in females. Indeed, FFM rep- resents the distribution volume of most cytotoxic chemo- therapy drugs. In 2,115 cancer patients, the individual variations in FFM could change by up to three times the distribution volume of the chemotherapy drug per body area unit [5]. Thus, administering the same doses of chemotherapy drugs to a patient with a low FFM compared to a patient with a normal FFM would increase the risk of chemotherapy toxicity [5]. These data suggest that FFM loss could have a direct impact on the clinical outcome of cancer patients. Decreasing chemotherapy doses in case of FFM loss could contribute to improving cancer patients� prognosis through the improvement of the tolerance of chemotherapy. These findings justify the systematic evaluation of body composition in all cancer patients in order to detect FFM loss, tailor chemotherapy doses according to FFM values, and then improve the efficacy- tolerance and cost-efficiency ratios of the therapeutic strategies [93]. Body composition evaluation should also be used to tailor the doses of drugs which are calculated based on patients� weight, e.g. corticosteroids, immuno-suppressors (infliximab, azathioprine or methotrexate), or sedatives (propofol).
 The implementation of body composition evaluation in routine care presents a challenge for the next decades. Indeed the concomitant increases in elderly subjects and patients with chronic diseases and cancer, and in the prevalence of overweight and obesity in the population, will increase the number of patients nutritionally at risk or undernourished, particularly those with sarcopenic obesity. Body composition evaluation should be used to improve the screening of undernutrition in hospitalized patients. The results of body composition should be based on the same principle as BMI calculation, towards the systematic normalization for body height of FFM (FFMI) and FM [FM (kg)/height (m)2 = FM index] [94]. The results could be expressed according to previously de- scribed percentiles of healthy subjects [95, 96]. Body com- position evaluation should be performed at the different stages of the disease, during the course of treatments and the rehabilitation phase. Such repeated evaluations of body composition could allow assessment of the nutritional status, adjusting the calculation of energy needs as kilocalories/kilogram FFM, following the efficacy of
The implementation of body composition evaluation in routine care presents a challenge for the next decades. Indeed the concomitant increases in elderly subjects and patients with chronic diseases and cancer, and in the prevalence of overweight and obesity in the population, will increase the number of patients nutritionally at risk or undernourished, particularly those with sarcopenic obesity. Body composition evaluation should be used to improve the screening of undernutrition in hospitalized patients. The results of body composition should be based on the same principle as BMI calculation, towards the systematic normalization for body height of FFM (FFMI) and FM [FM (kg)/height (m)2 = FM index] [94]. The results could be expressed according to previously de- scribed percentiles of healthy subjects [95, 96]. Body com- position evaluation should be performed at the different stages of the disease, during the course of treatments and the rehabilitation phase. Such repeated evaluations of body composition could allow assessment of the nutritional status, adjusting the calculation of energy needs as kilocalories/kilogram FFM, following the efficacy of 




