Psoriasis Abstract
Psoriasis is a common T-cell-mediated immune disorder characterized by circumscribed, red, thickened plaques with an overlying silver-white scale. It occurs worldwide, although the incidence is lower in warmer, sunnier climates. The primary cause of psoriasis is unknown. During an active disease state, an underlying inflammatory mechanism is frequently involved. Many conventional treatments focus on suppressing symptoms associated with psoriasis and have significant side effects. This article reviews several of the researched natural approaches to psoriasis treatment, while addressing its underlying cause. (Altern Med Rev 2007;12(4):319-330)
Introduction
Recent genetic and immunological advances have greatly increased understanding of the pathogenesis of psoriasis as a chronic, immune-mediated inflammatory disorder. The primary immune defect in psoriasis appears to be an increase in cell signaling via chemokines and cytokines that act on upregulated gene expression and cause hyper-proliferation of keratinocytes. A new understanding of this complex disease has catalyzed the development of targeted biological treatments. These revolutionary therapies are not without potential risk, however. A review of alternative natural therapies provides some options for increasing safety and efficacy in the management of psoriasis. Psoriasis � Pathophysiology, Conventional, and Alternative Approaches to Treatment Michael Traub, ND, and Keri Marshall MS, ND
Epidemiology
The prevalence of psoriasis varies widely depending on ethnicity. Psoriasis occurs most commonly in Caucasians, with an estimated occurrence of 60 cases per 100,000/year in this population. Its prevalence in the United States is 2-4 percent, although it is rare or absent in Native American and certain African-American populations. While common in Japan, it is much less common in China, with an estimated incidence of 0.3 percent. The prevalence in the general population of Northern Europe and Scandinavia is 1.5-3 percent. Women and men are equally affected by this condition. The observation that latitude affects prevalence is most likely related to the beneficial effect of sunlight on the disease.1 Although psoriasis can occur at any age, the mean age of onset for chronic plaque psoriasis is estimated at 33 years, with 75 percent of cases initiated before age 46.2 The age of onset appears to be slightly earlier in women than men. Longitudinal studies suggest spontaneous remission may occur in about one-third of patients with psoriasis.3
Pathophysiology
Until recently psoriasis was considered a disorder of epidermal keratinocytes; however, it is now recognized primarily as an immune-mediated disorder. In order to properly understand the immune dysfunction present in psoriasis, it is imperative to understand the normal immune response of skin. Skin is a primary lymphoid organ with an effective immunological surveillance system equipped with antigen presenting cells, cytokine synthesizing keratinocytes, epidermotropic T cells, dermal capillary endothelial cells, draining nodes, mast cells, tissue macrophages, granulocytes, fibroblasts, and non-Langerhans cells. Skin also has lymph nodes and circulating T lymphocytes. Together these cells communicate by means of cytokine secretion and respond accordingly via stimulation by bacteria, chemical, ultraviolet (UV) light, and other irritating factors. The primary cytokine released in response to antigen presentation is tumor necrosis factor-alpha (TNF-?). Generally, this is a controlled process unless the insult to the skin is prolonged, in which case imbalanced cytokine production leads to a pathological state such as psoriasis.
Debate continues whether psoriasis is an autoimmune disorder or a T-helper 1 (Th1) immune dysfunction. T-cell activation, TNF-?, and dendritic cells are pathogenic factors stimulated in response to a triggering factor, such as a physical injury, inflammation, bacteria, virus, or withdrawal of corticosteroid medication. Initially, immature dendritic cells in the epidermis stimulate T-cells from lymph nodes in response to as yet unidentified antigen stimulation. The lymphocytic infiltrate in psoriasis is predominately CD4 and CD8 T cells. Adhesion molecules that promote leukocyte adherence are highly expressed in psoriatic lesions.4 After T cells receive primary stimulation and activation, a resulting synthesis of mRNA for interleukin-2 (IL-2) occurs, resulting in a subsequent increase in IL-2 receptors. Psoriasis is considered a Th1-dominant disease due to the increase in cytokines of the Th1 pathway � interferon gamma (IFN-?), IL-2, and interleukin 12 (IL-12) � found in psoriatic plaques.
The increased IL-2 from activated T cells and IL-12 from Langerhans cells ultimately regulate genes that code for the transcription of cytokines such as IFN-?, TNF-?, and IL-2, responsible for differentiation, maturation, and proliferation of T cells into memory effector cells. Ultimately, T cells migrate to the skin, where they accumulate around dermal blood vessels. These are the first in a series of immunologic changes that result in the formation of acute psoriatic lesions. Because the above-described immune response is a somewhat normal response to antigen stimulation, it remains unclear why the T-cell activation that occurs, followed by subsequent migration of leukocytes into the epidermis and dermis, creates accelerated cellular proliferation. Upregulated gene regulation may be a causative factor. Vascular endothelial growth factor (VEGF) and interleukin-8 released from keratinocytes may contribute to the vascularization seen in psoriasis.5
Dendritic cells appear to be involved in the pathogenesis of psoriasis. One type of dendritic cell involved is the Langerhans cells, the outermost sentinel of the immune system that recognizes and captures antigens, migrates to local lymph nodes, and presents them to T cells. The activation of T lymphocytes releases pro-inflammatory cytokines such as TNF-? that lead to keratinocyte proliferation. This hyperproliferative response decreases epidermal transit time (the approximate time it takes for normal maturation of skin cells) from 28 days to 2-4 days and produces the typical erythematous scaly plaques of psoriasis. This understanding of pathogenic mechanisms has led to the development and therapeutic use of TNF-? blocking agents.
About 30 percent of individuals with psoriasis have a family history of the disease in a first- or seconddegree relative. At least nine chromosomal susceptibility loci have been elucidated (PSORS1-9). HLA-Cw6 is a major determinant of phenotypic expression. An association with the PSORS has been found with functional polymorphisms in modifier genes that mediate inflammation (e.g., TNF-?) and vascular growth (e.g., VEGF).6
It is known that psoriasis develops in bone marrow transplant recipients from donors with psoriasis, clears in recipients from donors without psoriasis, and that immunosuppressive drugs are effective in reducing psoriasis.7,8 Given the genetic predisposition to this disease, what can be done to reduce the genetic expression besides resorting to immunosuppressive therapies? A naturopathic approach consists of dietary modification,�therapeutic fasting, omega-3 supplementation, topical natural medicines, herbal medicine, and stress management.
Pizzorno and Murray propose the above-mentioned �unidentified antigens� result from incomplete protein digestion, increased intestinal permeability, and food allergies; bowel toxemia (endotoxins); impaired liver detoxification; bile acid deficiencies; alcohol consumption; excessive consumption of animal fats; nutrient deficiencies (vitamins A and E, zinc, and selenium); and stress.9 These hypotheses, although plausible, have not been adequately tested.
Co-Morbidities
Psoriasis is associated with several co-morbidities, including decreased quality of life, depression, increased cardiovascular risk, type 2 diabetes mellitus, metabolic syndrome, cancer, Crohn�s disease, and psoriatic arthritis. It remains unclear whether cancers, in particular skin cancer and lymphoma, are related to psoriasis or to its treatment. Phototherapy and immunosuppressive therapy can increase the risk of non-melanoma skin cancer, for example.10
Of particular concern is the observed link between psoriasis and cardiovascular disease. Evidence indicates psoriasis is an independent risk factor for cardiovascular disease.11 Dyslipidemia, coronary calcification, increased highly sensitive C-reactive protein (CRP), decreased folate, and hyperhomocysteinemia are found with significantly higher frequency in psoriasis patients.12 Inflammation is the common theme underlying both conditions, characterized by the presence of pro-inflammatory cytokines and endothelial activation.
The inflammatory processes underlying psoriasis also suggest the possibility of omega-3 fatty acid, folate, and vitamin B12 deficiencies, which are also often found in cardiovascular disease.13 High homocysteine and decreased folate levels correlate with Psoriasis Area and Severity Index (PASI). A rapid skin cell turnover rate in psoriasis may result in increased folate utilization and subsequent deficiency.14 The author of one study concludes: �Dietary supplementation of folic acid, B6, and B12 appears reasonable in psoriasis patients, particularly those with elevated homocysteine, low folate and additional cardiovascular risk factors.�15
Psoriatic arthritis is a clinical condition occurring in 25 percent of individuals afflicted with psoriasis.16 In approximately 10 percent of this population, the arthritic symptoms precede the skin lesions. Psoriatic arthritis often presents as seronegative inflammatory arthritis, with a classic presentation consisting of oligoarthritis, distal interphalangeal joint involvement, dactylitis (inflammation of the digits), and calcaneal inflammation.
Opinions conflict whether the skin condition and arthritis represent a differing manifestation of the same disease. Genetic evidence, immunological studies, and treatment response variability suggest they may be two different conditions, perhaps with similar underlying inflammation and immune irregularity.17,18
Although palmoplantar pustulosis (PP) is often described as a subtype of psoriasis, different demographics and genetic analysis suggests a different etiology than psoriasis. On appearance, PP has yellowbrown sterile pustules that appear on palms and soles. Only 25 percent of those affected report chronic plaque psoriasis. PP occurs more frequently in women (9:1/ female:male) and 95 percent of affected people have a current or previous history of smoking. As a result, PP may be considered a co-morbid condition rather than a distinct form of psoriasis.19
Diagnostic Criteria
Psoriasis is classified into several subtypes, with the chronic plaque (psoriasis vulgaris) form comprising approximately 90 percent of cases. Sharply demarcated erythematous silvery scaling plaques occur most commonly on the extensor surface of the elbows, knees, scalp, sacral, and groin regions. Other involved areas include the ears, glans penis, perianal region, and sites of repeated trauma. An active inflammatory case of psoriasis can demonstrate the Koebner phenomenon in which new lesions form at a site of trauma or pressure.
In the future, chronic plaque psoriasis might be found to consist of several related conditions with distinct phenotypical and genotypical characteristics, providing an explanation for its variable response to therapy, especially with biologic agents.
Inverse psoriasis occurs in intertriginous sites and skin folds and is red, shiny, and usually without scaling. Sebopsoriasis, which is often confused with seborrheic dermatitis, is characterized by greasy scales�in the eyebrows, nasolabial folds, and postauricular and presternal areas.
Acute guttate psoriasis occurs in children, adolescents, and young adults approximately two weeks after an acute beta-hemolytic streptococcal infection, such as tonsillitis or pharyngitis, or a viral infection. It manifests as an erythematous, papular eruption with lesions less than 1 cm in diameter on the trunk and extremities. Acute guttate psoriasis is usually self-limited, resolving within 3-4 months. One study indicated only one-third of individuals with guttate psoriasis develop classic plaque psoriasis.20
Pustular psoriasis (von Zumbusch) is also an acute psoriatic eruption. The patient presents with fever and small, monomorphic, painful, sterile pustules, often precipitated by an intercurrent infection or the abrupt withdrawal of systemic or superpotent topical steroids. It can be localized to the palms and soles (palmar-plantar psoriasis) or it can be generalized and potentially life-threatening.
Erythrodermic psoriasis, also life threatening, involves the entire body surface and can result in hypothermia, hypoalbuminemia, anemia, infection, and high-output cardiac failure.
Psoriatic nail disease occurs in approximately 50 percent of psoriasis patients and most commonly manifests as pitting. Other nail changes can include onycholysis, discoloration, thickening, and dystrophy.
Risk Factors
Development of psoriasis involves interaction of multiple genetic risk factors with environmental factors, such as beta-hemolytic streptococcal infection, HIV, stress, and medications (e.g., beta-blockers and lithium). As previously mentioned, folate and vitamin B12 deficiency can also predispose. In addition, there is evidence that alcoholism, cigarette smoking, obesity, type 2 diabetes mellitus, and metabolic syndrome increase risk for developing psoriasis.
With the exception of VEGF, no biomarkers have been found as reliable predictors of psoriasis activity. CRP, soluble adhesion molecules, and soluble cytokine receptors have been investigated but do not correlate with severity.21
Conventional Treatment
Conventional treatment of psoriasis is based on the degree of severity. Mild and limited psoriasis treatment includes topical corticosteroids, tars, anthralin, calcipotriene (a vitamin D3 analog), tazarotene (a retinoid), and phototherapy. Physicians can set realistic expectations for therapy, giving the patient control over the disease without expectation of complete cure. Scalp psoriasis usually responds to salicylic acid shampoos.
Narrow-band UVB is less effective but safer than psoralen plus ultraviolet A (PUVA), which carries with it an increased risk of skin cancer. Sun exposure is another form of phototherapy. UV exposure reduces antigen presenting and affects cell signaling, favoring development of T-helper 2 (Th2) immune responses. Antigen-presenting Langerhans cells are decreased in both number and function.22
A topical combination of calcipotriene and betamethasone (Taclonex�) has shown greater efficacy in severe psoriasis than monotherapy with either alone.23
Patient compliance must be considered when developing a treatment plan. The use of less messy topical solution and foam preparations of topical corticosteroids and calcipotriene (compared to ointments and creams) can improve compliance.
Systemic treatment of severe psoriasis usually involves the use of oral retinoids, methotrexate, cyclosporine, and biological agents that can significantly impact other bodily systems.
The oral retinoid acitretin is teratogenic and is converted to etretinate with concomitant alcohol ingestion. Etretinate has a longer half-life and is more teratogenic than acitretin. Female patients must use two forms of birth control and must not become pregnant for at least three years after treatment. Because of possible interaction with oral contraceptives, St. John�s wort (Hypericum perfoliatum) should be avoided. Other adverse effects include mucocutaneous effects, elevated triglycerides, alopecia, and hepatitis. Treatment with acitretin requires frequent monitoring of blood counts, comprehensive metabolic profiles, and urinalysis. Strategies to reduce acitretin toxicity include intermittent use, reduction of maintenance dose to every other day or every third day, combination treatment with PUVA or topical calcipotriene, low-fat diet, aerobic exercise, fish oil supplementation, and as stated above, alcohol avoidance.
Methotrexate (MTX) is the most commonly used systemic agent for psoriasis and, because it has been available for 35 years, most dermatologists are comfortable with its use. Methotrexate inhibits dihydrofolate reductase (resulting in a deficiency of active folic acid) and induces adenosine A1, a potent anti-inflammatory agonist. Its mechanism of action may be even more complex, evidenced by the fact that caffeine inhibits MTX�s anti-inflammatory effects in rheumatoid arthritis but not in psoriasis or psoriatic arthritis.24 The most common serious adverse effects of MTX are myelosuppression and liver fibrosis. While myelosuppression does not frequently occur, patients using MTX often report symptoms of headache, fatigue, and nausea. Folate supplementation reduces the incidence of megaloblastic anemia, hepatotoxicity, and gastrointestinal intolerance. Although folic acid and folinic acid appear to be equally effective, folic acid is more cost effective.25 However, a recent double-blind study of 22 psoriasis patients stable on long-term MTX therapy revealed folic acid reduced MTX�s efficacy in controlling psoriasis. Patients were randomly assigned to receive 5 mg/day folic acid or placebo for 12 weeks. The mean PASI increased (worsened) in the folic acid group, from 6.4 at baseline to 10.8 at 12 weeks. In the placebo group, the mean PASI fell from 9.8 at baseline to 9.2 at 12 weeks (p<0.05 for the difference in the change between groups).26
Cyclosporine, a potent and toxic drug, is sometimes considered for cases not controlled with acitretin, PUVA, or MTX, but is contraindicated in patients with abnormal renal function, poorly controlled hypertension, hepatic dysfunction, or immunosuppression. Prolonged use inevitably results in renal damage. Blood pressure and creatinine monitoring is essential.
Biological agents block T-cell activation and TNF-?. Alefacept (Amevive�) interferes with T-cell activation and reduces circulating CD 45 RO+ T cells. This drug is a fusion protein of the Fc receptor of human IgG1 and LFA3, a co-stimulatory ligand, which interacts with CD2 on the surface of T-cells. CD4 cells must be monitored weekly during treatment with this agent.
Efalizumab (Raptiva�) is a humanized antibody to CD11 that interferes with T-cell trafficking into inflamed tissues and prevents T-cell activation. Although it is rapidly effective, rebound may occur.
TNF-? blockers downregulate proinflammatory gene expression and reverse the psoriatic phenotype. Etanercept (Enbrel�) is a fusion protein directed against soluble TNF-?. Infliximab (Remicade�) is a mouse/human chimeric monoclonal antibody against soluble and cell-bound TNF-?, while adalimumab (Humira�) is a human monoclonal antibody against TNF-?. These TNF-? inhibitors are administered by injection and have been associated with the induction of various autoimmune phenomena. Like TNF-? itself, TNF-? inhibitors can have both proinflammatory and anti-inflammatory activities. Just because a particular agent blocks TNF-?, it does not necessarily benefit psoriasis. If a patient is genetically predisposed to overproducing TNF-?, blocking it may not be sufficient to produce benefit.27 Possible risks of TNF-? blockers include reactivation of latent tuberculosis, hepatotoxicity, lymphoma, and congestive heart failure.
Challenges that remain with biologics for psoriasis include: (1) understanding the predominant mechanism in psoriasis and psoriatic arthritis; (2) understanding different patient responses to therapy; (3) predicting clinical response before or early in therapy; (4) developing oral, inhaled, and topical formulations; and (5) determining whether treatment alters longterm outcome.
Fumaric acid is the primary psoriasis therapy in Germany. It decreases T-cell dependent cytokines, but is not as effective as other conventional treatments, and carries a high risk of toxicity and gastrointestinal intolerance.
Providing rotational and combination therapies increases efficacy and decreases toxicity of treatment. The future may bring stem-cell therapy and gene-based therapies, including �antisense� treatments that directly inhibit psoriasis-specific genes. However, the adverse effects and toxicity of conventional psoriasis treatments necessitate safer and effective natural treatments that can be used as alternatives or in an integrative fashion.
Natural Treatments For Psoriasis
Diet
An evidence-based approach suggests psoriasis, essentially an inflammatory disorder, should benefit from an anti-inflammatory diet, identification, elimination and/or rotation of allergenic foods, and therapeutic fasting.28-30 Although there is no published data on food allergy avoidance, many psoriasis patients show increased sensitivity to gluten and their psoriasis symptoms improve on a gluten-free diet.31 Measurement of antibodies to tissue transglutaminase and gliadin can help identify this subgroup. Evidence also suggests maintaining a healthy weight benefits psoriasis patients, since psoriasis positively correlates with increased body mass index.32
The balance between proinflammatory and anti-inflammatory eicosanoids is influenced in large part by the type of dietary fatty acids consumed. An antiinflammatory diet consists basically of an emphasis on �good fats� (cold water fish, nuts, seeds, olive oil, other high quality oils), whole grains, legumes, vegetables, and fruits and the avoidance of �bad fats� (saturated animal fats, trans fats, fried and processed foods, poor quality oils) and refined carbohydrates. In addition, an excessive amount of omega-6 fatty acids in the diet can contribute to an inflammatory response.33 The primary sources of dietary omega-6 oils are vegetable oils such as corn, soy, safflower, and sunflower, while the primary sources of arachidonic acid are meat, eggs, and dairy.
Prostaglandin E2 (PGE2) is a prominent eicosanoid derived from the omega-6 fatty acid arachidonic acid. A dominant action of PGE2 as a messenger molecule is to enhance sensitivity in pain neurons, increase swelling, and constrict blood vessels. Over-consumption of omega-6 oils provides excess substrate for the synthesis of PGE2, which drives an aggressive and sustained inflammatory response. Prostaglandin E3 (PGE3) is�derived from the omega-3 fatty acid, eicosapentaenoic acid (EPA). Higher levels of PGE3 reduce sensitivity to pain, relax blood vessels, increase blood flow, and support the body�s natural anti-inflammatory response (Figure 1).
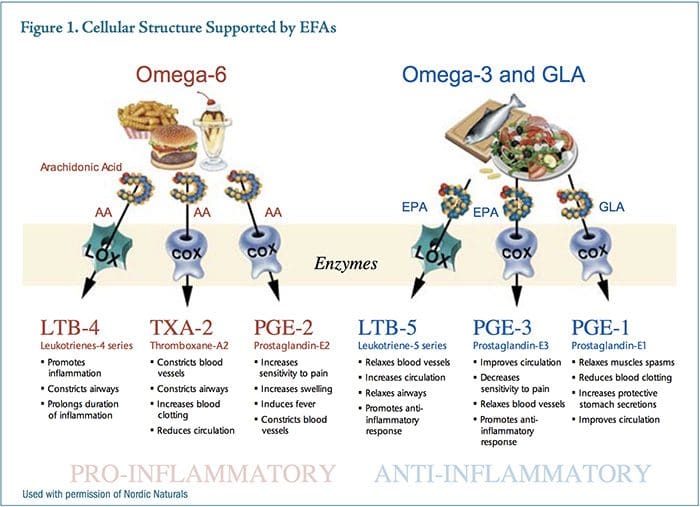
While both PGE2 and PGE3 are necessary for proper homeostasis, the relative amounts of these competing messenger molecules either contribute to or mitigate chronic inflammatory syndromes. EPA is thought to act by competing with arachidonic acid for binding sites on cyclooxygenase-2 (COX-2), producing a less potent inflammatory mediator, therefore reducing inflammation.34
Prior to the Industrial Revolution, there were no significant sources of omega-6 vegetable oils in the diet. Most cultures consumed diets low in these oils and high in fish or range-fed beef or bison higher in omega-3s, creating a ratio of omega-6:omega-3 that was approximately 3:1. The Industrial Revolution brought with it the knowledge and tools to refine vegetable oils, resulting in a rapid shift in dietary habits for most Western cultures. The ratio of omega-6:omega-3 was quickly pushed toward the current estimate of as high as 11:1 omega-6:omega-3.35 The human body has not been able to genetically adapt to this dramatic shift in fatty acid consumption.
Many modern cultures consume copious amounts of vegetable oils, mostly in processed foods. For example, soy oil production for food consumption increased 1,000-fold between 1909 and 1999.36 In addition, livestock, poultry, and farmed fish are being fed cornmeal and soy-based feed, which raises the omega-6 content of the meat and fish. When farm animals are raised on grass, worms, or other natural diets, the tissues are naturally higher in omega-3 fatty acids.37
The beef industry touts �marbling� in finished beef products, which is due to the corn and soy feed. Corn- and soy-fed cattle have a higher omega-6 fatty acid content compared to grass-fed cattle. While grassfed cattle can contain up to 4-percent omega-3 fatty acids, corn-fed cattle typically contains 0.5-percent omega-3s.37
The standard American diet supplies an average omega-6:omega-3 ratio of approximately 11:1. A vegetarian-based diet may put an individual at risk for�eating high amounts of vegetable oils and soy products, and low amounts of fish, which can tip the balance toward a pro-inflammatory state. Reducing dietary vegetable oils and increasing the omega-3 fats EPA and docosahexaenoic acid (DHA) by consuming fatty fish such as cod, salmon, mackerel, and sardines can benefit individuals experiencing chronic inflammatory conditions.33
Several herbs used as seasonings, including turmeric, red pepper, cloves, ginger, cumin, anise, fennel, basil, rosemary, garlic, and pomegranate, can block nuclear factor-kappaB (NF?B) activation of inflammatory cytokines.38
Dietary approaches that modify fatty acid intake can influence the eicosanoid profile in such a way that inflammatory processes such as arachidonic acid production and T-cell activation are dampened, while cytokines such as interleukin-4 (the primary cytokine responsible for stimulating a Th2 immune response) are upregulated.34
Nutritional Supplementation
Essential Fatty Acids
Essential fatty acids (EFAs) influence the pathophysiology of psoriasis in three ways: first, EFAs impact the kinetics of cell membranes; second, EFAs impact dermal and epidermal blood flow via improved endothelial function; and third, EFAs act as an immunomodulating agent through their impact on eicosanoids. EFAs are used as basic substrates in the development of the phospholipid bi-layer in virtually every cell in the human body, including the dermis and epidermis. They create structural integrity that regulates fluidity, which impacts cell transport, messenger binding, and cell communication. Omega-3 fatty acids can act both directly and indirectly on endothelial function by reducing mononuclear cell cytokines such as IL-1 and TNF?, 39 decreasing formation of chemo-attractant protein platelet-derived growth factor (PDGF), increasing bioavailability of nitric oxide, and reducing expression of adhesion molecules. The cumulative effect modulating these bioactive mediators is to prevent vascularization, or new blood vessel growth within the psoriatic plaque, while simultaneously allowing improved perfusion of dermal tissue.
Components of both natural and acquired immunity, including the production of key immune modulators, can be affected by omega-3 and -6 fatty acid intake, as discussed above. Immunomodulatory effects of omega-3 fatty acids include suppression of lymphoproliferation, CD4+ cells, antigen presentation, adhesion molecule presentation, Th1 and Th2 responses, and pro-inflammatory cytokine production.34
Several studies have demonstrated the benefit of intravenous or oral supplementation of fish oil for psoriasis.40-42 In a study by Mayser et al, intravenous infusions of omega-3 fatty acids led to an increase in the anti-inflammatory leukotriene B5 (LTB5) within 4-7 days of starting treatment, when compared to control patients.43 In this trial, patients received either an omega-3 or omega-6 preparation twice daily for 10 days. No side effects were noted.
EPA competes with arachidonic acid (AA) for 5-lipoxygenase and produces LTB5, which is only one-tenth as potent as the inflammatory mediator leukotriene B4 (LTB4). Levels of LTB4 have been shown to be elevated in psoriatic plaques and demonstrate chemotactic properties necessary for infiltration of leukocyte and keratinocyte proliferation.43
Ziboh�s review article on omega-3s and psoriasis references six studies conducted using oral fish oil supplementation with mixed results. Unfortunately, original references cannot be found. Two studies were double-blind and placebo-controlled, using 1.8 g EPA and DHA over courses of eight weeks and 12 weeks. The eight-week study demonstrated benefit in itching, scaling, and erythema, while the 12-week study showed no benefit.44
Three open studies were conducted, providing 10-18 g EPA and DHA daily for eight weeks. All studies showed improvement, with two studies demonstrating mild-to-moderate and one study demonstrating moderate-to-excellent improvement in scaling, itching, and lesion thickness. One open study combined with a low-fat diet showed a significant reduction in psoriatic symptoms.44,45
Several studies have explored the use of topical fish oil at varying EPA concentrations. Some studies reported benefits, including a reduction in plaque thickness and scaling.46,47 In one study by Puglia et al, fish oil extracts and ketoprofen were applied topically to�psoriatic lesions, with an observed reduction in erythema.48 The most significant drawback to topical fish oil application is compliance due to the odor.
Fish oil has also proven to be beneficial in autoimmune joint conditions such as rheumatoid arthritis (RA).49 While fish oil supplementation has not been used in clinical trials for the treatment of psoriatic arthritis, it may be beneficial in treating this condition, which has many similarities to RA, including a common underlying inflammatory mechanism and immune dysfunction.
Folate
Methotrexate therapy results in a folate deficiency. As mentioned above, in patients receiving MTX for psoriasis, folate supplementation reduced the incidence of hepatotoxicity and gastrointestinal intolerance but might impair the efficacy of MTX.24 When supplementing with folic acid or the active forms, folinic acid or 5-methyltetrahydrofolate, the recommended dose is 1-5 mg/day.
Bioactive Whey Protein Isolate
XP-828L is a novel dietary supplement made of a protein extract derived from bovine whey that has recently been shown to be beneficial in psoriasis.50,51 The bioactive profile of XP-828L is likely due to the presence of growth factors, immunoglobulins, and active peptides found in this specific whey extract. An in vitro study demonstrated XP-828L has immune-regulating effects, including inhibiting the production of Th1 cytokines such as IFN-g and IL-2, which may make it effective in treating T-helper 1-related disorders, such as psoriasis.52
An open-label study was conducted on 11 adult patients with chronic, stable plaque psoriasis on two percent or more of total body surface area. Study participants received 5 g twice daily of XP-828L for 56 days. Evaluations using PASI and Physician�s Global Assessment (PGA) scores were made on the initial screening day and again on days 1, 28, and 56. At the conclusion of the study, seven of the 11 subjects had a reduced PASI score that ranged from 9.5 percent to 81.3 percent.50 The results of a larger double-blind,�placebo-controlled study of 84 individuals with mildto-moderate psoriasis showed XP-828L (5 g/day for 56 days) significantly reduced the PGA score compared to placebo (p<0.05). No adverse affects were noted from any study participants in either study.50,51
Vitamin D
It has been established that patients with disseminated psoriasis have significantly decreased serum levels of the biologically active form of vitamin D, 1-alpha,25-dihydroxyvitamin D3 (1-?,25(OH)2D3; calcitriol) compared to age- and sex-matched controls and also compared to patients with moderate psoriasis.53 Whether this is a contributing factor to psoriasis or a result of the disorder has not been elucidated.
Keratinocytes in the epidermis convert 7-dehydrocholesterol to vitamin D3 in the presence of UVB. Sunlight, UVB phototherapy, oral calcitriol, and topical vitamin D analogs are effective therapy for psoriasis due to vitamin D�s anti-proliferative and pro-differentiating actions on keratinocytes.54-56
Calcitriol-binding to vitamin D receptors (VDR) in the skin modulates the expression of a large number of genes including cell cycle regulators, growth factors, and their receptors. Polymorphisms of the VDR gene are associated with psoriasis and may predispose to the development of psoriasis and resistance to calcipotriol therapy, as well as contribute to liver dysfunction in patients with psoriasis.57
Given vitamin D�s importance in psoriasis, cancer, inflammatory diseases, and other conditions, it has been suggested by some investigators that recommendations for sun protection and skin cancer prevention may need to be re-evaluated to allow for sufficient vitamin D status. A recent study showed abundant sun exposure in a sample of adults in Hawaii did not necessarily ensure vitamin D adequacy, which points to the need for vitamin D supplementation to achieve optimal blood levels.58
Studies have demonstrated that oral vitamin D can be safely taken in daily doses of up to 5,000 IU, with some experts recommending up to 10,000 IU daily to correct a deficiency.59-61 Oral and topical vitamin D, sunlight, and UVB phototherapy have shown considerable efficacy in the treatment of psoriasis.56
Topical Treatments Of Psoriasis
Several topical treatments for psoriasis may provide benefit, including calcipotriene (Dovonex�; a synthetic vitamin D3 analogue), Berberis aquifolium cream (10%)62 (Psoriaflora�; Relieva�), curcumin gel (1%), Aloe vera, and a flavonoid-rich salve (Flavsalve�).
Curcumin gel yielded 90-percent resolution of plaques in 50 percent of patients within 2-6 weeks; the remainder of the study subjects showed 50- to 85-percent improvement. Curcumin was found to be twice as effective as calcipotriol cream (which generally takes three months to exert its full effect). The mechanism of curcumin is as a selective phosphorylase kinase inhibitor, thereby reducing inflammation through inhibition of NF?B.63
A controlled trial of Aloe vera extract cream (0.5%) in 60 patients for 4-12 months demonstrated a significant clearing of psoriatic plaques (82.8%) compared to placebo (7.7%) (p<0.001). In addition, the PASI decreased to a mean of 2.2.64
The scaliness of psoriasis benefits from the use of emollients. Intercellular lipids such as ceramides (lipid molecules composed of fatty acids and sphingosine) play an important role in the regulation of skin-water barrier homeostasis and water-holding capacity. It has been shown that ceramides are decreased in the psoriatic epidermis. Newer ceramide-containing emollients (e.g., CeraVe�, Mimyx�, Aveeno Eczema Care) have shown benefit in psoriasis and may improve skin barrier function and decrease water loss.65
Botanical Influences
A Chinese herbal formula (Herose� Psoria Capsule) has demonstrated safety and efficacy in the treatment of severe plaque psoriasis.66 Herose consists of rhizoma Zingiberis, radix Salviae miltiorrhizae, radix Astragali, ramulus Cinnamomi, radix Paeoniae alba, radix Codonopsis pilosula, and semen Coicis. In an openlabel trial, 15 subjects took four Herose capsules (450 mg each) three times daily for 10 months. The investigator evaluated the PASI and therapeutic response to Herose for each patient. The formula is intended for warming the yang and promoting blood circulation.
Lifestyle Interventions
Lifestyle factors such as cigarette smoking and alcohol consumption are associated with severity of psoriasis.67 Physical activity and outdoor activities (taking precautions not to sunburn) are beneficial.68 Bathing and sunbathing at the Dead Sea for four weeks resulted in a decrease of PASI of 81.5 percent, a 78-percent decrease in keratinocyte hyperplasia, and almost total elimination of T lymphocytes from the epidermis, with a low number remaining in the dermis.69
Stress management can benefit individuals with psoriasis. Subjects who listened to a guided meditation tape while undergoing phototherapy cleared four times faster than those who received phototherapy only, as judged by two independent dermatologists. Psoriasis status was assessed in three ways: direct inspection by clinic nurses; direct inspection by physicians blinded to the patient�s study condition (tape or no-tape); and blinded physician evaluation of photographs of psoriasis lesions. Four sequential indicators of skin status were monitored during the study: a First Response Point, a Turning Point, a Halfway Point, and a Clearing Point. Subjects in the tape groups reached the Halfway Point (p= 0.013) and the Clearing Point (p=0.033) significantly more rapidly than those in the no-tape condition, for both UVB and PUVA treatments.70 Finally, psychotherapy can be an essential adjunct for individuals with persistent unresolved psychological issues such as anxiety, depression, and the psychosocial stress of this chronic skin disease.
Discussion
Psoriasis is characterized by T-cell activation that releases pro-inflammatory cytokines such as TNF-?, leading to keratinocyte proliferation and the typical skin lesions of psoriasis.
The conventional approach to psoriasis consists of utilizing topical and/or oral corticosteroids, other immunosuppressant drugs, oral retinoids, UV light, and several (not necessarily novel, having been used previously for Crohn�s and RA) biological agents. Although these treatments can be highly effective at controlling the disease, none are universally safe and effective, and each carries a considerable risk profile.
There is some evidence for the use of dietary modification and fish oil to decrease inflammation in psoriasis. More research is warranted to clarify the use�of these and various topical botanical therapies and lifestyle interventions for improving clinical symptoms, decreasing the phenotypic expression of psoriasis, and providing safe and effective treatments.