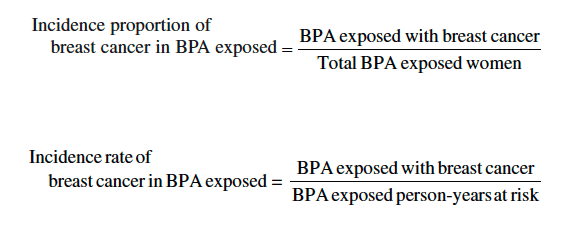
1. Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, Bray F:
International variation in prostate cancer incidence and mortality rates.
Eur Urol 2012, 61:1079�1092.
2. Masko EM, Allott EH, Freedland SJ: The relationship between nutrition and
prostate cancer: is more always better? Eur Urol 2013, 63:810�820.
3. Mavropoulos JC, Isaacs WB, Pizzo SV, Freedland SJ: Is there a role for a
low-carbohydrate ketogenic diet in the management of prostate cancer?
Urology 2006, 68:15�18.
4. Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W,
Aronson WJ, Cohen P, Hwang D, Peterson B, Fields T, Pizzo SV, Isaacs WB:
Carbohydrate restriction, prostate cancer growth, and the insulin-like
growth factor axis. Prostate 2008, 68:11�19.
5. Mavropoulos JC: Buschemeyer WC 3rd, Tewari AK, Rokhfeld D, Pollak M,
Zhao Y, Febbo PG, Cohen P, Hwang D, Devi G, Demark-Wahnefried W,
Westman EC, Peterson BL, Pizzo SV, Freedland SJ: The effects of varying
dietary carbohydrate and fat content on survival in a murine LNCaP
prostate cancer xenograft model. Cancer Prev Res (Phila Pa) 2009,
2:557�565.
6. Masko EM, Thomas JA 2nd, Antonelli JA, Lloyd JC, Phillips TE, Poulton SH,
Dewhirst MW, Pizzo SV, Freedland SJ: Low-carbohydrate diets and
prostate cancer: how low is �low enough�? Cancer Prev Res (Phila) 2010,
3:1124�1131.
7. Drake I, Sonestedt E, Gullberg B, Ahlgren G, Bjartell A, Wallstrom P, Wirf�lt E:
Dietary intakes of carbohydrates in relation to prostate cancer risk: a
prospective study in the Malmo Diet and Cancer cohort. Am J Clin Nutr
2012, 96:1409�1418.
8. Zhang J, Shen C, Wang L, Ma Q, Xia P, Qi M, Yang M, Han B: Metformin
inhibits epithelial-mesenchymal transition in prostate cancer cells:
Involvement of the tumor suppressor miR30a and its target gene SOX4.
Biochem Biophys Res Commun 2014, 452:746�752.
9. Lee SY, Song CH, Xie YB, Jung C, Choi HS, Lee K: SMILE upregulated by
metformin inhibits the function of androgen receptor in prostate cancer
cells. Cancer Lett 2014, 354:390�397.
10. Demir U, Koehler A, Schneider R, Schweiger S, Klocker H: Metformin antitumor
effect via disruption of the MID1 translational regulator complex
and AR downregulation in prostate cancer cells. BMC Cancer 2014, 14:52.
11. Margel D: Metformin to prevent prostate cancer: a call to unite. Eur Urol
2014. doi:10.1016/j.eururo.2014.05.012. [Epub ahead of time]
12. Margel D, Urbach DR, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, Fleshner
N: Metformin use and all-cause and prostate cancer-specific mortality
among men with diabetes. J Clin Oncol 2013, 31:3069�3075.
13. Tseng CH: Metformin significantly reduces incident prostate cancer risk
in Taiwanese men with type 2 diabetes mellitus. Eur J Cancer 2014,
50:2831�2837.
14. Joshua AM, Zannella VE, Downes MR, Bowes B, Hersey K, Koritzinsky M,
Schwab M, Hofmann U, Evans A, van der Kwast T, Trachtenberg J, Finelli A,
Fleshner N, Sweet J, Pollak M: A pilot �window of opportunity�
neoadjuvant study of metformin in localised prostate cancer. Prostate
Cancer Prostatic Dis 2014, 17:252�258.
15. Rothermundt C, Hayoz S, Templeton AJ, Winterhalder R, Strebel RT, Bartschi
D, Pollak M, Lui L, Endt K, Schiess R, R�schoff JH, Cathomas R, Gillessen S:
Metformin in Chemotherapy-naive Castration-resistant Prostate Cancer:
A Multicenter Phase 2 Trial (SAKK 08/09). Eur Urol 2014, 66:468�474.
16. Allott EH, Abern MR, Gerber L, Keto CJ, Aronson WJ, Terris MK, Kane CJ,
Amling CL, Cooperberg MR, Moorman PG, Freedland SJ: Metformin does
not affect risk of biochemical recurrence following radical
prostatectomy: results from the SEARCH database. Prostate Cancer
Prostatic Dis 2013, 16:391�397.
17. Rieken M, Kluth LA, Xylinas E, Fajkovic H, Becker A, Karakiewicz PI, Herman
M, Lotan Y, Seitz C, Schramek P, Remzi M, Loidl W, Pummer K, Lee RK,
Faison T, Scherr DS, Kautzky-Willer A, Bachmann A, Tewari A, Shariat SF:
Association of diabetes mellitus and metformin use with biochemical
recurrence in patients treated with radical prostatectomy for prostate
cancer. World J Urol 2014, 32:999�1005.
18. Margel D, Urbach D, Lipscombe LL, Bell CM, Kulkarni G, Austin PC, Fleshner
N: Association between metformin use and risk of prostate cancer and
its grade. J Natl Cancer Inst 2013, 105:1123�1131.
19. Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A:
Metformin therapy and risk of cancer in patients with type 2 diabetes:
systematic review. PLoS One 2013, 8:e71583.
20. Kaushik D, Karnes RJ, Eisenberg MS, Rangel LJ, Carlson RE, Bergstralh EJ:
Effect of metformin on prostate cancer outcomes after radical
prostatectomy. Urol Oncol 2014, 32:43 e41�47.
21. Bensimon L, Yin H, Suissa S, Pollak MN, Azoulay L: The use of metformin in
patients with prostate cancer and the risk of death. Cancer Epidemiol
Biomarkers Prev 2014, 23:2111�2118.
22. Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, van Veldhoven K,
Sacerdote C, Ashby D, Vineis P, Tzoulaki I, Ioannidis JP: Metformin does not
affect cancer risk: a cohort study in the U.K. Clinical Practice Research
Datalink analyzed like an intention-to-treat trial. Diabetes Care 2014,
37:2522�2532.
23. Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F,
Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK,
Wei M, Cohen P, Crimmins EM, Longo VD: Low protein intake is associated
with a major reduction in IGF-1, cancer, and overall mortality in the 65
and younger but not older population. Cell Metab 2014, 19:407�417.
24. Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC,
Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N,
Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ: The
ratio of macronutrients, not caloric intake, dictates cardiometabolic
health, aging, and longevity in ad libitum-fed mice. Cell Metab 2014,
19:418�430.
25. Richman EL, Stampfer MJ, Paciorek A, Broering JM, Carroll PR, Chan JM:
Intakes of meat, fish, poultry, and eggs and risk of prostate cancer
progression. Am J Clin Nutr 2010, 91:712�721.
26. Joshi AD, John EM, Koo J, Ingles SA, Stern MC: Fish intake, cooking
practices, and risk of prostate cancer: results from a multi-ethnic
case�control study. Cancer Causes Control 2012, 23:405�420.
27. Joshi AD, Corral R, Catsburg C, Lewinger JP, Koo J, John EM, Ingles SA,
Stern MC: Red meat and poultry, cooking practices, genetic susceptibility
and risk of prostate cancer: results from a multiethnic case�control
study. Carcinogenesis 2012, 33:2108�2118.
28. Catsburg C, Joshi AD, Corral R, Lewinger JP, Koo J, John EM, Ingles SA,
Stern MC: Polymorphisms in carcinogen metabolism enzymes, fish
intake, and risk of prostate cancer. Carcinogenesis 2012, 33:1352�1359.
29. Pettersson A, Kasperzyk JL, Kenfield SA, Richman EL, Chan JM, Willett WC,
Stampfer MJ, Mucci LA, Giovannucci EL: Milk and dairy consumption
among men with prostate cancer and risk of metastases and prostate
cancer death. Cancer Epidemiol Biomarkers Prev 2012, 21:428�436.
30. Deneo-Pellegrini H, Ronco AL, De Stefani E, Boffetta P, Correa P,
Mendilaharsu M, Acosta G: Food groups and risk of prostate cancer: a
case�control study in Uruguay. Cancer Causes Control 2012, 23:1031�1038.
31. Park SY, Murphy SP, Wilkens LR, Stram DO, Henderson BE, Kolonel LN:
Calcium, vitamin D, and dairy product intake and prostate cancer risk:
the Multiethnic Cohort Study. Am J Epidemiol 2007, 166:1259�1269.
32. Song Y, Chavarro JE, Cao Y, Qiu W, Mucci L, Sesso HD, Stampfer MJ,
Giovannucci E, Pollak M, Liu S, Ma J: Whole milk intake is associated with
prostate cancer-specific mortality among U.S. male physicians. J Nutr Feb
2013, 143:189�196.
33. Young NJ, Metcalfe C, Gunnell D, Rowlands MA, Lane JA, Gilbert R, Avery
KN, Davis M, Neal DE, Hamdy FC, Donovan J, Martin RM, Holly JM: A crosssectional
analysis of the association between diet and insulin-like growth
factor (IGF)-I, IGF-II, IGF-binding protein (IGFBP)-2, and IGFBP-3 in men in
the United Kingdom. Cancer Causes Control 2012, 23:907�917.
34. Christensen MJ, Quiner TE, Nakken HL, Lephart ED, Eggett DL, Urie PM:
Combination effects of dietary soy and methylselenocysteine in a mouse
model of prostate cancer. Prostate 2013, 73:986�995.
35. Bosland MC, Kato I, Zeleniuch-Jacquotte A, Schmoll J, Enk Rueter E,
Melamed J, Kong MX, Macias V, Kajdacsy-Balla A, Lumey LH, Xie H, Gao W,
Walden P, Lepor H, Taneja SS, Randolph C, Schlicht MJ, Meserve-Watanabe
H, Deaton RJ, Davies JA: Effect of soy protein isolate supplementation on
biochemical recurrence of prostate cancer after radical prostatectomy: a
randomized trial. JAMA 2013, 310:170�178.
36. Chiyomaru T, Yamamura S, Fukuhara S, Yoshino H, Kinoshita T, Majid S, Saini
S, Chang I, Tanaka Y, Enokida H, Seki N, Nakagawa M, Dahiya R: Genistein
inhibits prostate cancer cell growth by targeting miR-34a and oncogenic
HOTAIR. PLoS One 2013, 8:e70372.
37. Zhang S, Wang Y, Chen Z, Kim S, Iqbal S, Chi A, Ritenour C, Wang YA, Kucuk
O, Wu D: Genistein enhances the efficacy of cabazitaxel chemotherapy
in metastatic castration-resistant prostate cancer cells. Prostate 2013,
73:1681�1689.38. van Die MD, Bone KM, Williams SG, Pirotta MV: Soy and soy isoflavones in
prostate cancer: a systematic review and meta-analysis of randomized
controlled trials. BJU Int 2014, 113:E119�E130.
39. Hamilton-Reeves JM, Banerjee S, Banerjee SK, Holzbeierlein JM, Thrasher JB,
Kambhampati S, Keighley J, Van Veldhuizen P: Short-term soy isoflavone
intervention in patients with localized prostate cancer: a randomized,
double-blind, placebo-controlled trial. PLoS One 2013, 8:e68331.
40. Pavese JM, Krishna SN, Bergan RC: Genistein inhibits human prostate
cancer cell detachment, invasion, and metastasis. Am J Clin Nutr 2014,
100:431S�436S.
41. Gonzalez-Menendez P, Hevia D, Rodriguez-Garcia A, Mayo JC, Sainz RM:
Regulation of GLUT transporters by flavonoids in androgen-sensitive and
-insensitive prostate cancer cells. Endocrinology 2014, 155:3238�3250.
42. Hirata H, Hinoda Y, Shahryari V, Deng G, Tanaka Y, Tabatabai ZL, Dahiya R:
Genistein downregulates onco-miR-1260b and upregulates sFRP1 and
Smad4 via demethylation and histone modification in prostate cancer
cells. Br J Cancer 2014, 110:1645�1654.
43. Handayani R, Rice L, Cui Y, Medrano TA, Samedi VG, Baker HV, Szabo NJ,
Shiverick KT: Soy isoflavones alter expression of genes associated with
cancer progression, including interleukin-8, in androgen-independent
PC-3 human prostate cancer cells. J Nutr 2006, 136:75�82.
44. Travis RC, Allen NE, Appleby PN, Price A, Kaaks R, Chang-Claude J, Boeing H,
Aleksandrova K, Tj�nneland A, Johnsen NF, Overvad K, Ram�n Quir�s J,
Gonz�lez CA, Molina-Montes E, S�nchez MJ, Larra�aga N, Casta�o JM,
Ardanaz E, Khaw KT, Wareham N, Trichopoulou A, Karapetyan T, Rafnsson
SB, Palli D, Krogh V, Tumino R, Vineis P, Bueno-de-Mesquita HB, Stattin P,
Johansson M, et al: Prediagnostic concentrations of plasma genistein and
prostate cancer risk in 1,605 men with prostate cancer and 1,697
matched control participants in EPIC. Cancer Causes Control 2012,
23:1163�1171.
45. Jackson MD, McFarlane-Anderson ND, Simon GA, Bennett FI, Walker SP:
Urinary phytoestrogens and risk of prostate cancer in Jamaican men.
Cancer Causes Control 2010, 21:2249�2257.
46. Lazarevic B, Hammarstr�m C, Yang J, Ramberg H, Diep LM, Karlsen SJ,
Kucuk O, Saatcioglu F, Task�n KA, Svindland A: The effects of short-term
genistein intervention on prostate biomarker expression in patients with
localised prostate cancer before radical prostatectomy. Br J Nutr 2012,
108:2138�2147.
47. Epstein MM, Kasperzyk JL, Mucci LA, Giovannucci E, Price A, Wolk A,
H�kansson N, Fall K, Andersson SO, Andr�n O: Dietary fatty acid intake and
prostate cancer survival in Orebro County, Sweden. Am J Epidemiol 2012,
176:240�252.
48. Kobayashi N, Barnard RJ, Said J, Hong-Gonzalez J, Corman DM, Ku M,
Doan NB, Gui D, Elashoff D, Cohen P, Aronson WJ: Effect of low-fat diet on
development of prostate cancer and Akt phosphorylation in the Hi-Myc
transgenic mouse model. Cancer Res 2008, 68:3066�3073.
49. Ngo TH, Barnard RJ, Cohen P, Freedland S, Tran C, deGregorio F, Elshimali
YI, Heber D, Aronson WJ: Effect of isocaloric low-fat diet on human
LAPC-4 prostate cancer xenografts in severe combined immunodeficient
mice and the insulin-like growth factor axis. Clin Cancer Res 2003,
9:2734�2743.
50. Huang M, Narita S, Numakura K, Tsuruta H, Saito M, Inoue T, Horikawa Y,
Tsuchiya N, Habuchi T: A high-fat diet enhances proliferation of
prostate cancer cells and activates MCP-1/CCR2 signaling. Prostate 2012,
72:1779�1788.
51. Chang SN, Han J, Abdelkader TS, Kim TH, Lee JM, Song J, Kim KS, Park JH,
Park JH: High animal fat intake enhances prostate cancer progression
and reduces glutathione peroxidase 3 expression in early stages of
TRAMP mice. Prostate 2014, 74:1266�1277.
52. Bidoli E, Talamini R, Bosetti C, Negri E, Maruzzi D, Montella M, Franceschi S,
La Vecchia C: Macronutrients, fatty acids, cholesterol and prostate cancer
risk. Ann Oncol 2005, 16:152�157.
53. Park SY, Murphy SP, Wilkens LR, Henderson BE, Kolonel LN: Fat and meat
intake and prostate cancer risk: the multiethnic cohort study. Int J Cancer
2007, 121:1339�1345.
54. Wallstrom P, Bjartell A, Gullberg B, Olsson H, Wirfalt E: A prospective study
on dietary fat and incidence of prostate cancer (Malmo, Sweden).
Cancer Causes Control 2007, 18:1107�1121.
55. Crowe FL, Key TJ, Appleby PN, Travis RC, Overvad K, Jakobsen MU,
Johnsen NF, Tj�nneland A, Linseisen J, Rohrmann S, Boeing H, Pischon T,
Trichopoulou A, Lagiou P, Trichopoulos D, Sacerdote C, Palli D, Tumino R,
Krogh V, Bueno-de-Mesquita HB, Kiemeney LA, Chirlaque MD, Ardanaz E,
S�nchez MJ, Larra�aga N, Gonz�lez CA, Quir�s JR, Manjer J, Wirf�lt E, Stattin
P, et al: Dietary fat intake and risk of prostate cancer in the European
Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr 2008,
87:1405�1413.
56. Ohwaki K, Endo F, Kachi Y, Hattori K, Muraishi O, Nishikitani M, Yano E:
Relationship between dietary factors and prostate-specific antigen in
healthy men. Urol Int 2012, 89:270�274.
57. Bassett JK, Severi G, Hodge AM, MacInnis RJ, Gibson RA, Hopper JL,
English DR, Giles GG: Plasma phospholipid fatty acids, dietary fatty acids
and prostate cancer risk. Int J Cancer 2013, 133:1882�1891.
58. Richman EL, Kenfield SA, Chavarro JE, Stampfer MJ, Giovannucci EL, Willett
WC, Chan JM: Fat intake after diagnosis and risk of lethal prostate cancer
and all-cause mortality. JAMA Intern Med 2013, 173:1318�1326.
59. Williams CD, Whitley BM, Hoyo C, Grant DJ, Iraggi JD, Newman KA, Gerber
L, Taylor LA, McKeever MG, Freedland SJ: A high ratio of dietary n-6/n-3
polyunsaturated fatty acids is associated with increased risk of prostate
cancer. Nutr Res 2011, 31:1�8.
60. Chua ME, Sio MC, Sorongon MC, Dy JS: Relationship of dietary intake of
omega-3 and omega-6 fatty acids with risk of prostate cancer
development: a meta-analysis of prospective studies and review of
literature. Prostate Cancer 2012, 2012:826254.
61. Berquin IM, Edwards IJ, Kridel SJ, Chen YQ: Polyunsaturated fatty acid
metabolism in prostate cancer. Cancer Metastasis Rev 2011, 30:295�309.
62. Aronson WJ, Kobayashi N, Barnard RJ, Henning S, Huang M, Jardack PM, Liu
B, Gray A, Wan J, Konijeti R, Freedland SJ, Castor B, Heber D, Elashoff D, Said
J, Cohen P, Galet C: Phase II prospective randomized trial of a low-fat diet
with fish oil supplementation in men undergoing radical prostatectomy.
Cancer Prev Res (Phila) 2011, 4:2062�2071.
63. Hughes-Fulford M, Li CF, Boonyaratanakornkit J, Sayyah S: Arachidonic acid
activates phosphatidylinositol 3-kinase signaling and induces gene
expression in prostate cancer. Cancer Res 2006, 66:1427�1433.
64. Moreel X, Allaire J, Leger C, Caron A, Labonte ME, Lamarche B, Julien P,
Desmeules P, T�tu B, Fradet V: Prostatic and dietary omega-3 fatty acids
and prostate cancer progression during active surveillance. Cancer Prev
Res (Phila) 2014, 7:766�776.
65. Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, Spencer D, Berry D,
Steward W, Dennison A: The effect of omega-3 FAs on tumour angiogenesis
and their therapeutic potential. Eur J Cancer 2009, 45:2077�2086.
66. Gu Z, Suburu J, Chen H, Chen YQ: Mechanisms of omega-3 polyunsaturated
fatty acids in prostate cancer prevention. Biomed Res Int 2013, 2013:824563.
67. Lloyd JC, Masko EM, Wu C, Keenan MM, Pilla DM, Aronson WJ, Chi JT,
Freedland SJ: Fish oil slows prostate cancer xenograft growth relative to
other dietary fats and is associated with decreased mitochondrial and
insulin pathway gene expression. Prostate Cancer Prostatic Dis 2013,
16:285�291.
68. Williams CM, Burdge G: Long-chain n-3 PUFA: plant v. marine sources.
Proc Nutr Soc 2006, 65:42�50.
69. Galet C, Gollapudi K, Stepanian S, Byrd JB, Henning SM, Grogan T, Elashoff
D, Heber D, Said J, Cohen P, Aronson WJ: Effect of a low-fat fish oil diet
on proinflammatory eicosanoids and cell-cycle progression score in
men undergoing radical prostatectomy. Cancer Prev Res (Phila) 2014,
7:97�104.
70. Bosire C, Stampfer MJ, Subar AF, Park Y, Kirkpatrick SI, Chiuve SE, Hollenbeck
AR, Reedy J: Index-based dietary patterns and the risk of prostate cancer
in the NIH-AARP diet and health study. Am J Epidemiol 2013, 177:504�513.
71. Aronson WJ, Barnard RJ, Freedland SJ, Henning S, Elashoff D, Jardack PM,
Cohen P, Heber D, Kobayashi N: Growth inhibitory effect of low fat diet
on prostate cancer cells: results of a prospective, randomized dietary
intervention trial in men with prostate cancer. J Urol 2010, 183:345�350.
72. Brouwer IA, Geleijnse JM, Klaasen VM, Smit LA, Giltay EJ, de Goede J,
Heijboer AC, Kromhout D, Katan MB: Effect of alpha linolenic acid
supplementation on serum prostate specific antigen (PSA): results from
the alpha omega trial. PLoS One 2013, 8:e81519.
73. Chua ME, Sio MC, Sorongon MC, Morales ML Jr: The relevance of serum
levels of long chain omega-3 polyunsaturated fatty acids and prostate
cancer risk: A meta-analysis. Can Urol Assoc J 2013, 7:E333�E343.
74. Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X,
Ratliff TL, Cheng JX: Cholesteryl ester accumulation induced by PTEN loss
and PI3K/AKT activation underlies human prostate cancer
aggressiveness. Cell Metab 2014, 19:393�406.
75. Sun Y, Sukumaran P, Varma A, Derry S, Sahmoun AE, Singh BB: Cholesterolinduced
activation of TRPM7 regulates cell proliferation, migration,
and viability of human prostate cells. Biochim Biophys Acta 1843,
2014:1839�1850.
76. Murai T: Cholesterol lowering: role in cancer prevention and treatment.
Biol Chem 2014. doi:10.1515/hsz-2014-0194. [Epub ahead of time]
77. Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR: Cholesterol
targeting alters lipid raft composition and cell survival in prostate cancer
cells and xenografts. J Clin Invest 2005, 115:959�968.
78. Mostaghel EA, Solomon KR, Pelton K, Freeman MR, Montgomery RB:
Impact of circulating cholesterol levels on growth and intratumoral
androgen concentration of prostate tumors. PLoS One 2012,
7:e30062.
79. Morote J, Celma A, Planas J, Placer J, de Torres I, Olivan M, Carles J,
Revent�s J, Doll A: Role of serum cholesterol and statin use in the risk of
prostate cancer detection and tumor aggressiveness. Int J Mol Sci 2014,
15:13615�13623.
80. Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK,
Amling CL, Freedland SJ: Postoperative statin use and risk of biochemical
recurrence following radical prostatectomy: results from the Shared
Equal Access Regional Cancer Hospital (SEARCH) database. BJU Int 2014,
114:661�666.
81. Jespersen CG, Norgaard M, Friis S, Skriver C, Borre M: Statin use and risk of
prostate cancer: A Danish population-based case�control study,
1997�2010. Cancer Epidemiol 2014, 38:42�47.
82. Meyers CD, Kashyap ML: Pharmacologic elevation of high-density
lipoproteins: recent insights on mechanism of action and atherosclerosis
protection. Curr Opin Cardiol 2004, 19:366�373.
83. Xia P, Vadas MA, Rye KA, Barter PJ, Gamble JR: High density lipoproteins
(HDL) interrupt the sphingosine kinase signaling pathway. A possible
mechanism for protection against atherosclerosis by HDL. J Biol Chem
1999, 274:33143�33147.
84. Kotani K, Sekine Y, Ishikawa S, Ikpot IZ, Suzuki K, Remaley AT: High-density
lipoprotein and prostate cancer: an overview. J Epidemiol 2013,
23:313�319.
85. Soni MG, Thurmond TS, Miller ER 3rd, Spriggs T, Bendich A, Omaye ST:
Safety of vitamins and minerals: controversies and perspective. Toxicol
Sci 2010, 118:348�355.
86. Neuhouser ML, Barnett MJ, Kristal AR, Ambrosone CB, King I, Thornquist M,
Goodman G: (n-6) PUFA increase and dairy foods decrease prostate
cancer risk in heavy smokers. J Nutr 2007, 137:1821�1827.
87. Karppi J, Kurl S, Laukkanen JA, Kauhanen J: Serum beta-carotene in relation
to risk of prostate cancer: the Kuopio Ischaemic Heart Disease Risk
Factor study. Nutr Cancer 2012, 64:361�367.
88. Margalit DN, Kasperzyk JL, Martin NE, Sesso HD, Gaziano JM, Ma J, Stampfer
MJ, Mucci LA: Beta-carotene antioxidant use during radiation therapy
and prostate cancer outcome in the Physicians� Health Study. Int J Radiat
Oncol Biol Phys 2012, 83:28�32.
89. Roswall N, Larsen SB, Friis S, Outzen M, Olsen A, Christensen J, Dragsted LO,
Tj�nneland A: Micronutrient intake and risk of prostate cancer in a
cohort of middle-aged, Danish men. Cancer Causes Control 2013,
24:1129�1135.
90. Gilbert R, Metcalfe C, Fraser WD, Donovan J, Hamdy F, Neal DE, Lane JA,
Martin RM: Associations of circulating retinol, vitamin E, and 1,25-
dihydroxyvitamin D with prostate cancer diagnosis, stage, and grade.
Cancer Causes Control 2012, 23:1865�1873.
91. Bistulfi G, Foster BA, Karasik E, Gillard B, Miecznikowski J, Dhiman VK,
Smiraglia DJ: Dietary folate deficiency blocks prostate cancer progression
in the TRAMP model. Cancer Prev Res (Phila) 2011, 4:1825�1834.
92. Collin SM: Folate and B12 in prostate cancer. Adv Clin Chem 2013,
60:1�63.
93. Tio M, Andrici J, Cox MR, Eslick GD: Folate intake and the risk of prostate
cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic
Dis 2014, 17:213�219.
94. Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, Armitage J,
Manson JE, Hankey GJ, Spence JD, Galan P, B�naa KH, Jamison R, Gaziano
JM, Guarino P, Baron JA, Logan RF, Giovannucci EL, den Heijer M, Ueland
PM, Bennett D, Collins R, Peto R, B-Vitamin Treatment Trialists’ Collaboration:
Effects of folic acid supplementation on overall and site-specific cancer
incidence during the randomised trials: meta-analyses of data on 50,000
individuals. Lancet 2013, 381:1029�1036.
95. Verhage BA, Cremers P, Schouten LJ, Goldbohm RA, van den Brandt PA:
Dietary folate and folate vitamers and the risk of prostate cancer
in The Netherlands Cohort Study. Cancer Causes Control 2012,
23:2003�2011.
96. Tavani A, Malerba S, Pelucchi C, Dal Maso L, Zucchetto A, Serraino D, Levi F,
Montella M, Franceschi S, Zambon A, La Vecchia C: Dietary folates and
cancer risk in a network of case�control studies. Ann Oncol 2012,
23:2737�2742.
97. Moreira DM, Banez LL, Presti JC Jr, Aronson WJ, Terris MK, Kane CJ, Amling
CL, Freedland SJ: High serum folate is associated with reduced
biochemical recurrence after radical prostatectomy: results from the
SEARCH Database. Int Braz J Urol 2013, 39:312�318. discussion 319.
98. Han YY, Song JY, Talbott EO: Serum folate and prostate-specific antigen in
the United States. Cancer Causes Control 2013, 24:1595�1604.
99. Rycyna KJ, Bacich DJ, O’Keefe DS: Opposing roles of folate in prostate
cancer. Urology 2013, 82:1197�1203.
100. Gilbert R, Martin RM, Beynon R, Harris R, Savovic J, Zuccolo L, Bekkering GE,
Fraser WD, Sterne JA, Metcalfe: Associations of circulating and dietary
vitamin D with prostate cancer risk: a systematic review and dose�
response meta-analysis. Cancer Causes Control 2011, 22:319�340.
101. Schenk JM, Till CA, Tangen CM, Goodman PJ, Song X, Torkko KC, Kristal AR,
Peters U, Neuhouser ML: Serum 25-hydroxyvitamin d concentrations and
risk of prostate cancer: results from the Prostate Cancer Prevention Trial.
Cancer Epidemiol Biomarkers Prev 2014, 23:1484�1493.
102. Schwartz GG: Vitamin D, in blood and risk of prostate cancer: lessons
from the Selenium and Vitamin E Cancer Prevention Trial and the
Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev 2014,
23:1447�1449.
103. Giangreco AA, Vaishnav A, Wagner D, Finelli A, Fleshner N, Van der Kwast T,
Vieth R, Nonn L: Tumor suppressor microRNAs, miR-100 and -125b, are
regulated by 1,25-dihydroxyvitamin D in primary prostate cells and in
patient tissue. Cancer Prev Res (Phila) 2013, 6:483�494.
104. Hollis BW, Marshall DT, Savage SJ, Garrett-Mayer E, Kindy MS, Gattoni-Celli S:
Vitamin D3 supplementation, low-risk prostate cancer, and health
disparities. J Steroid Biochem Mol Biol 2013, 136:233�237.
105. Sha J, Pan J, Ping P, Xuan H, Li D, Bo J, Liu D, Huang Y: Synergistic effect
and mechanism of vitamin A and vitamin D on inducing apoptosis of
prostate cancer cells. Mol Biol Rep 2013, 40:2763�2768.
106. Chandler PD, Giovannucci EL, Scott JB, Bennett GG, Ng K, Chan AT, Hollis
BW, Emmons KM, Fuchs CS, Drake BF: Null association between Vitamin D
and PSA levels among black men in a Vitamin D supplementation trial.
Cancer Epidemiol Biomarkers Prev 2014, 23:1944�1947.
107. Skaaby T, Husemoen LL, Thuesen BH, Pisinger C, Jorgensen T, Roswall N,
Larsen SC, Linneberg A: Prospective population-based study of the
association between serum 25-hydroxyvitamin-D levels and the
incidence of specific types of cancer. Cancer Epidemiol Biomarkers Prev
2014, 23:1220�1229.
108. Holt SK, Kolb S, Fu R, Horst R, Feng Z, Stanford JL: Circulating levels of
25-hydroxyvitamin D and prostate cancer prognosis. Cancer Epidemiol
2013, 37:666�670.
109. Wong YY, Hyde Z, McCaul KA, Yeap BB, Golledge J, Hankey GJ, Flicker L:
In older men, lower plasma 25-hydroxyvitamin D is associated with
reduced incidence of prostate, but not colorectal or lung cancer.
PLoS One 2014, 9:e99954.
110. Xu Y, Shao X, Yao Y, Xu L, Chang L, Jiang Z, Lin Z: Positive association
between circulating 25-hydroxyvitamin D levels and prostate cancer risk:
new findings from an updated meta-analysis. J Cancer Res Clin Oncol
2014, 140:1465�1477.
111. Meyer HE, Robsahm TE, Bjorge T, Brustad M, Blomhoff R: Vitamin D, season,
and risk of prostate cancer: a nested case�control study within
Norwegian health studies. Am J Clin Nutr 2013, 97:147�154.
112. Kristal AR, Till C, Song X, Tangen CM, Goodman PJ, Neuhauser ML, Schenk
JM, Thompson IM, Meyskens FL Jr, Goodman GE, Minasian LM, Parnes HL,
Klein EA: Plasma vitamin D and prostate cancer risk: results from the
Selenium and Vitamin E Cancer Prevention Trial. Cancer Epidemiol
Biomarkers Prev 2014, 23:1494�1504.
113. Weinstein SJ, Mondul AM, Kopp W, Rager H, Virtamo J, Albanes D:
Circulating 25-hydroxyvitamin D, vitamin D-binding protein and risk of
prostate cancer. Int J Cancer 2013, 132:2940�2947.
114. Guo Z, Wen J, Kan Q, Huang S, Liu X, Sun N, Li Z: Lack of association
between vitamin D receptor gene FokI and BsmI polymorphisms and�prostate cancer risk: an updated meta-analysis involving 21,756 subjects. Tumour Biol 2013, 34:3189�3200115. Wang L, Sesso HD, Glynn RJ, Christen WG, Bubes V, Manson JE, Buring JE,
Gaziano JM: Vitamin E and C supplementation and risk of cancer in men:
posttrial follow-up in the Physicians� Health Study II randomized trial.
Am J Clin Nutr 2014, 100:915�923.
116. Virtamo J, Taylor PR, Kontto J, Mannisto S, Utriainen M, Weinstein SJ,
Huttunen J, Albanes D: Effects of alpha-tocopherol and beta-carotene
supplementation on cancer incidence and mortality: 18-year
postintervention follow-up of the Alpha-tocopherol, Beta-carotene
Cancer Prevention Study. Int J Cancer 2014, 135:178�185.
117. Basu A, Imrhan V: Vitamin E and prostate cancer: is vitamin E succinate a
superior chemopreventive agent? Nutr Rev 2005, 63:247�251.
118. Lawson KA, Wright ME, Subar A, Mouw T, Hollenbeck A, Schatzkin A,
Leitzmann MF: Multivitamin use and risk of prostate cancer in the
National Institutes of Health-AARP Diet and Health Study. J Natl Cancer
Inst 2007, 99:754�764.
119. Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML,
Feigelson HS, Thun MJ: The American Cancer Society Cancer Prevention
Study II Nutrition Cohort: rationale, study design, and baseline
characteristics. Cancer 2002, 94:2490�2501.
120. Weinstein SJ, Peters U, Ahn J, Friesen MD, Riboli E, Hayes RB, Albanes D:
Serum alpha-tocopherol and gamma-tocopherol concentrations and
prostate cancer risk in the PLCO Screening Trial: a nested case�control
study. PLoS One 2012, 7:e40204.
121. Cui R, Liu ZQ, Xu Q: Blood alpha-tocopherol, gamma-tocopherol levels
and risk of prostate cancer: a meta-analysis of prospective studies.
PLoS One 2014, 9:e93044.
122. Major JM, Yu K, Weinstein SJ, Berndt SI, Hyland PL, Yeager M, Chanock S,
Albanes D: Genetic variants reflecting higher vitamin e status in men are
associated with reduced risk of prostate cancer. J Nutr May 2014,
144:729�733.
123. Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ,
Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther
PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE,
Meyskens FL Jr, Baker LH: Vitamin E and the risk of prostate cancer: the
Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011,
306:1549�1556.
124. Albanes D, Till C, Klein EA, Goodman PJ, Mondul AM, Weinstein SJ, aylor PR,
Parnes HL, Gaziano JM, Song X, Fleshner NE, Brown PH, Meyskens FL Jr,
Thompson IM: Plasma tocopherols and risk of prostate cancer in the
Selenium and Vitamin E Cancer Prevention Trial (SELECT). Cancer Prev Res
(Phila) 2014, 7:886�895.
125. Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, Thompson IM,
Meyskens FL Jr, Goodman GE, Minasian LM, Parnes HL, Lippman SM,
Klein EA: Baseline selenium status and effects of selenium and vitamin e
supplementation on prostate cancer risk. J Natl Cancer Inst 2014,
106:djt456.
126. Jamison JM, Gilloteaux J, Taper HS, Summers JL: Evaluation of the in vitro
and in vivo antitumor activities of vitamin C and K-3 combinations
against human prostate cancer. J Nutr 2001, 131:158S�160S.
127. Nimptsch K, Rohrmann S, Kaaks R, Linseisen J: Dietary vitamin K intake
in relation to cancer incidence and mortality: results from the
Heidelberg cohort of the European Prospective Investigation into
Cancer and Nutrition (EPIC-Heidelberg). Am J Clin Nutr 2010,
91:1348�1358.
128. Ma RW, Chapman K: A systematic review of the effect of diet in prostate
cancer prevention and treatment. J Hum Nutr Diet 2009, 22:187�199.
quiz 200�182.
129. Bristow SM, Bolland MJ, MacLennan GS, Avenell A, Grey A, Gamble GD, Reid
IR: Calcium supplements and cancer risk: a meta-analysis of randomised
controlled trials. Br J Nutr 2013, 110:1384�1393.
130. Williams CD, Whitley BM, Hoyo C, Grant DJ, Schwartz GG, Presti JC Jr, Iraggi
JD, Newman KA, Gerber L, Taylor LA, McKeever MG, Freedland SJ: Dietary
calcium and risk for prostate cancer: a case�control study among US
veterans. Prev Chronic Dis 2012, 9:E39.
131. Hori S, Butler E, McLoughlin J: Prostate cancer and diet: food for thought?
BJU Int 2011, 107:1348�1359.
132. Geybels MS, Verhage BA, van Schooten FJ, Goldbohm RA, van den Brandt
PA: Advanced prostate cancer risk in relation to toenail selenium levels.
J Natl Cancer Inst 2013, 105:1394�1401.
133. Singh RP, Agarwal R: Prostate cancer chemoprevention by silibinin: bench
to bedside. Mol Carcinog 2006, 45:436�442.
134. Ting H, Deep G, Agarwal R: Molecular mechanisms of silibinin-mediated
cancer chemoprevention with major emphasis on prostate cancer.
AAPS J 2013, 15:707�716.
135. Ting HJ, Deep G, Jain AK, Cimic A, Sirintrapun J, Romero LM, Cramer SD,
Agarwal C, Agarwal R: Silibinin prevents prostate cancer cell-mediated
differentiation of naive fibroblasts into cancer-associated fibroblast
phenotype by targeting TGF beta2. Mol Carcinog 2014. doi:10.1002/
mc.22135. [Epub ahead of time]
136. Goel A, Aggarwal BB: Curcumin, the golden spice from Indian saffron, is a
chemosensitizer and radiosensitizer for tumors and chemoprotector and
radioprotector for normal organs. Nutr Cancer 2010, 62:919�930.
137. Khan N, Adhami VM, Mukhtar H: Apoptosis by dietary agents for
prevention and treatment of prostate cancer. Endocr Relat Cancer 2010,
17:R39�R52.
138. Heber D: Pomegranate ellagitannins. In Herbal Medicine: Biomolecular and
Clinical Aspects. 2nd edition. Edited by Benzie IF, Wachtel-Galor S. Boca
Raton, FL: CRC Press; 2011.
139. Pantuck AJ, Leppert JT, Zomorodian N, Aronson W, Hong J, Barnard RJ,
Seeram N, Liker H, Wang H, Elashoff R, Heber D, Aviram M, Ignarro L,
Belldegrun A: Phase II study of pomegranate juice for men with rising
prostate-specific antigen following surgery or radiation for prostate
cancer. Clin Cancer Res 2006, 12:4018�4026.
140. Paller CJ, Ye X, Wozniak PJ, Gillespie BK, Sieber PR, Greengold RH, Stockton
BR, Hertzman BL, Efros MD, Roper RP, Liker HR, Carducci MA: A randomized
phase II study of pomegranate extract for men with rising PSA following
initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis
2013, 16:50�55.
141. Freedland SJ, Carducci M, Kroeger N, Partin A, Rao JY, Jin Y, Kerkoutian S,
Wu H, Li Y, Creel P, Mundy K, Gurganus R, Fedor H, King SA, Zhang Y,
Heber D, Pantuck AJ: A double-blind, randomized, neoadjuvant study of
the tissue effects of POMx pills in men with prostate cancer before
radical prostatectomy. Cancer Prev Res (Phila) 2013, 6:1120�1127.
142. Wang P, Aronson WJ, Huang M, Zhang Y, Lee RP, Heber D, Henning SM:
Green tea polyphenols and metabolites in prostatectomy tissue:
implications for cancer prevention. Cancer Prev Res (Phila) 2010,
3:985�993.
143. Kurahashi N, Sasazuki S, Iwasaki M, Inoue M, Tsugane S: Green tea
consumption and prostate cancer risk in Japanese men: a prospective
study. Am J Epidemiol 2008, 167:71�77.
144. McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA: Tea
polyphenols decrease serum levels of prostate-specific antigen,
hepatocyte growth factor, and vascular endothelial growth factor in
prostate cancer patients and inhibit production of hepatocyte growth
factor and vascular endothelial growth factor in vitro. Cancer Prev Res
(Phila) 2009, 2:673�682.
145. Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A:
Chemoprevention of human prostate cancer by oral administration of
green tea catechins in volunteers with high-grade prostate intraepithelial
neoplasia: a preliminary report from a one-year proof-of-principle study.
Cancer Res 2006, 66:1234�1240.
146. Fraser SP, Peters A, Fleming-Jones S, Mukhey D, Djamgoz MB: Resveratrol:
inhibitory effects on metastatic cell behaviors and voltage-gated Na(+)
channel activity in rat prostate cancer in vitro. Nutr Cancer 2014,
66:1047�1058.
147. Oskarsson A, Spatafora C, Tringali C, Andersson AO: Inhibition of CYP17A1
activity by resveratrol, piceatannol, and synthetic resveratrol analogs.
Prostate 2014, 74:839�851.
148. Ferruelo A, Romero I, Cabrera PM, Arance I, Andres G, Angulo JC: Effects of
resveratrol and other wine polyphenols on the proliferation, apoptosis
and androgen receptor expression in LNCaP cells. Actas Urol Esp Jul-Aug
2014, 38:397�404.
149. Osmond GW, Masko EM, Tyler DS, Freedland SJ, Pizzo S: In vitro and in vivo
evaluation of resveratrol and 3,5-dihydroxy-4?-acetoxy-trans-stilbene in
the treatment of human prostate carcinoma and melanoma. J Surg Res
2013, 179:e141�e148.
150. Baur JA, Sinclair DA: Therapeutic potential of resveratrol: the in vivo
evidence. Nat Rev Drug Discov 2006, 5:493�506.
151. Klink JC, Tewari AK, Masko EM, Antonelli J, Febbo PG, Cohen P, Dewhirst
MW, Pizzo SV, Freedland SJ: Resveratrol worsens survival in SCID mice with prostate cancer xenografts in a cell-line specific manner, through paradoxical effects on oncogenic pathways. Prostate 2013, 73:754�762.
152. Huang EC, Zhao Y, Chen G, Baek SJ, McEntee MF, Minkin S, Biggerstaff JP,
Whelan J: Zyflamend, a polyherbal mixture, down regulates class I and
class II histone deacetylases and increases p21 levels in castrate-resistant
prostate cancer cells. BMC Complement Altern Med 2014, 14:68.
153. Huang EC, McEntee MF, Whelan J: Zyflamend, a combination of herbal
extracts, attenuates tumor growth in murine xenograft models of
prostate cancer. Nutr Cancer 2012, 64:749�760.
154. Yan J, Xie B, Capodice JL, Katz AE: Zyflamend inhibits the expression and
function of androgen receptor and acts synergistically with bicalutimide
to inhibit prostate cancer cell growth. Prostate 2012, 72:244�252.
155. Kunnumakkara AB, Sung B, Ravindran J, Diagaradjane P, Deorukhkar A, Dey
S, Koca C, Tong Z, Gelovani JG, Guha S, Krishnan S, Aggarwal BB: Zyflamend
suppresses growth and sensitizes human pancreatic tumors to
gemcitabine in an orthotopic mouse model through modulation of
multiple targets. Int J Cancer 2012, 131:E292�E303.
156. Capodice JL, Gorroochurn P, Cammack AS, Eric G, McKiernan JM, Benson
MC, Stone BA, Katz AE: Zyflamend in men with high-grade prostatic
intraepithelial neoplasia: results of a phase I clinical trial. J Soc Integr
Oncol 2009, 7:43�51.
157. Rafailov S, Cammack S, Stone BA, Katz AE: The role of Zyflamend, an
herbal anti-inflammatory, as a potential chemopreventive agent against
prostate cancer: a case report. Integr Cancer Ther 2007, 6:74�76.
158. Askari F, Parizi MK, Jessri M, Rashidkhani B: Fruit and vegetable intake in
relation to prostate cancer in Iranian men: a case�control study.
Asian Pac J Cancer Prev 2014, 15:5223�5227.
159. Liu B, Mao Q, Cao M, Xie L: Cruciferous vegetables intake and risk of
prostate cancer: a meta-analysis. Int J Urol 2012, 19:134�141.
160. Richman EL, Carroll PR, Chan JM: Vegetable and fruit intake after
diagnosis and risk of prostate cancer progression. Int J Cancer 2012,
131:201�210.
161. Hsing AW, Chokkalingam AP, Gao YT, Madigan MP, Deng J, Gridley G,
Fraumeni JF Jr: Allium vegetables and risk of prostate cancer: a
population-based study. J Natl Cancer Inst 2002, 94:1648�1651.
162. Chan R, Lok K, Woo J: Prostate cancer and vegetable consumption.
Mol Nutr Food Res 2009, 53:201�216.
163. Thomas R, Williams M, Sharma H, Chaudry A, Bellamy P: A double-blind,
placebo-controlled randomised trial evaluating the effect of a
polyphenol-rich whole food supplement on PSA progression in men
with prostate cancer-the UK NCRN Pomi-T study. Prostate Cancer Prostatic
Dis 2014, 17:180�186.
164. Yang CM, Lu IH, Chen HY, Hu ML: Lycopene inhibits the proliferation of
androgen-dependent human prostate tumor cells through activation of
PPARgamma-LXRalpha-ABCA1 pathway. J Nutr Biochem 2012, 23:8�17.
165. Qiu X, Yuan Y, Vaishnav A, Tessel MA, Nonn L, van Breemen RB: Effects of
lycopene on protein expression in human primary prostatic epithelial
cells. Cancer Prev Res (Phila) 2013, 6:419�427.
166. Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW Jr, Clinton SK: Prostate
carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated
rats fed tomato powder, lycopene, or energy-restricted diets. J Natl
Cancer Inst 2003, 95:1578�1586.
167. Konijeti R, Henning S, Moro A, Sheikh A, Elashoff D, Shapiro A, Ku M,
Said JW, Heber D, Cohen P, Aronson WJ: Chemoprevention of prostate
cancer with lycopene in the TRAMP model. Prostate 2010, 70:1547�1554.
168. Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC: A prospective
study of tomato products, lycopene, and prostate cancer risk. J Natl
Cancer Inst 2002, 94:391�398.
169. Zu K, Mucci L, Rosner BA, Clinton SK, Loda M, Stampfer MJ, Giovannucci E:
Dietary lycopene, angiogenesis, and prostate cancer: a prospective
study in the prostate-specific antigen era. J Natl Cancer Inst 2014,
106:djt430.
170. Gann PH, Ma J, Giovannucci E, Willett W, Sacks FM, Hennekens CH, Stampfer
MJ: Lower prostate cancer risk in men with elevated plasma lycopene
levels: results of a prospective analysis. Cancer Res 1999, 59:1225�1230.
171. Kristal AR, Till C, Platz EA, Song X, King IB, Neuhouser ML, Ambrosone CB,
Thompson IM: Serum lycopene concentration and prostate cancer risk:
results from the Prostate Cancer Prevention Trial. Cancer Epidemiol
Biomarkers Prev 2011, 20:638�646.
172. Kirsh VA, Mayne ST, Peters U, Chatterjee N, Leitzmann MF, Dixon LB, Urban
DA, Crawford ED, Hayes RB: A prospective study of lycopene and tomato
product intake and risk of prostate cancer. Cancer Epidemiol Biomarkers
Prev 2006, 15:92�98.
173. Mariani S, Lionetto L, Cavallari M, Tubaro A, Rasio D, De Nunzio C, Hong
GM, Borro M, Simmaco M: Low prostate concentration of lycopene is
associated with development of prostate cancer in patients with highgrade
prostatic intraepithelial neoplasia. Int J Mol Sci 2014, 15:1433�1440.
174. Kucuk O, Sarkar FH, Djuric Z, Sakr W, Pollak MN, Khachik F, Banerjee M,
Bertram JS, Wood DP Jr: Effects of lycopene supplementation in patients
with localized prostate cancer. Exp Biol Med (Maywood) 2002, 227:881�885.
175. Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, van
Breemen R, Ashton D, Bowen PE: Oxidative DNA damage in prostate
cancer patients consuming tomato sauce-based entrees as a whole-food
intervention. J Natl Cancer Inst 2001, 93:1872�1879.
176. van Breemen RB, Sharifi R, Viana M, Pajkovic N, Zhu D, Yuan L, Yang Y,
Bowen PE, Stacewicz-Sapuntzakis M: Antioxidant effects of lycopene in
African American men with prostate cancer or benign prostate hyperplasia:
a randomized, controlled trial. Cancer Prev Res (Phila) 2011, 4:711�718.
177. Shafique K, McLoone P, Qureshi K, Leung H, Hart C, Morrison DS: Coffee
consumption and prostate cancer risk: further evidence for inverse
relationship. Nutr J 2012, 11:42.
178. Wilson KM, Kasperzyk JL, Rider JR, Kenfield S, van Dam RM, Stampfer MJ,
Giovannucci E, Mucci LA: Coffee consumption and prostate cancer risk
and progression in the Health Professionals Follow-up Study. J Natl
Cancer Inst 2011, 103:876�884.
179. Bosire C, Stampfer MJ, Subar AF, Wilson KM, Park Y, Sinha R: Coffee
consumption and the risk of overall and fatal prostate cancer in the
NIH-AARP Diet and Health Study. Cancer Causes Control 2013, 24:1527�1534.
180. Arab L, Su LJ, Steck SE, Ang A, Fontham ET, Bensen JT, Mohler JL: Coffee
consumption and prostate cancer aggressiveness among African and
Caucasian Americans in a population-based study. Nutr Cancer 2012,
64:637�642.
181. Phillips RL, Snowdon DA: Association of meat and coffee use with cancers
of the large bowel, breast, and prostate among Seventh-Day Adventists:
preliminary results. Cancer Res 1983, 43:2403 s�2408s.
182. Hsing AW, McLaughlin JK, Schuman LM, Bjelke E, Gridley G, Wacholder S,
Chien HT, Blot WJ: Diet, tobacco use, and fatal prostate cancer: results
from the Lutheran Brotherhood Cohort Study. Cancer Res 1990,
50:6836�6840.
183. Cao S, Liu L, Yin X, Wang Y, Liu J, Lu Z: Coffee consumption and risk of
prostate cancer: a meta-analysis of prospective cohort studies.
Carcinogenesis 2014, 35:256�261.
184. Nordmann AJ, Suter-Zimmermann K, Bucher HC, Shai I, Tuttle KR,
Estruch R, Briel M: Meta-analysis comparing Mediterranean to low-fat
diets for modification of cardiovascular risk factors. Am J Med 2011,
124:841�851. e842.
185. Kapiszewska M: A vegetable to meat consumption ratio as a relevant
factor determining cancer preventive diet. The Mediterranean versus
other European countries. Forum Nutr 2006, 59:130�153.
186. Kenfield SA, Dupre N, Richman EL, Stampfer MJ, Chan JM, Giovannucci EL:
Mediterranean diet and prostate cancer risk and mortality in the Health
Professionals Follow-up Study. Eur Urol 2014, 65:887�894.
187. Ambrosini GL, Fritschi L, de Klerk NH, Mackerras D, Leavy J: Dietary patterns
identified using factor analysis and prostate cancer risk: a case control
study in Western Australia. Ann Epidemiol 2008, 18:364�370.
188. Baade PD, Youlden DR, Krnjacki LJ: International epidemiology of prostate
cancer: geographical distribution and secular trends. Mol Nutr Food Res
2009, 53:171�184.
189. Muller DC, Severi G, Baglietto L, Krishnan K, English DR, Hopper JL, Giles GG:
Dietary patterns and prostate cancer risk. Cancer Epidemiol Biomarkers Prev
2009, 18:3126�3129.
190. Tseng M, Breslow RA, DeVellis RF, Ziegler RG: Dietary patterns and prostate
cancer risk in the National Health and Nutrition Examination Survey
Epidemiological Follow-up Study cohort. Cancer Epidemiol Biomarkers Prev
2004, 13:71�77.
191. Wu K, Hu FB, Willett WC, Giovannucci E: Dietary patterns and risk of
prostate cancer in U.S. men. Cancer Epidemiol Biomarkers Prev 2006,
15:167�171.
192. Daubenmier JJ, Weidner G, Marlin R, Crutchfield L, Dunn-Emke S, Chi C,
Gao B, Carroll P, Ornish D: Lifestyle and health-related quality of life of
men with prostate cancer managed with active surveillance. Urology
2006, 67:125�130.
193. Parsons JK, Newman VA, Mohler JL, Pierce JP, Flatt S, Marshall J: Dietary
modification in patients with prostate cancer on active surveillance: a
randomized, multicentre feasibility study. BJU Int 2008, 101:1227�1231.
194. Mosher CE, Sloane R, Morey MC, Snyder DC, Cohen HJ, Miller PE,
Demark-Wahnefried W: Associations between lifestyle factors and quality
of life among older long-term breast, prostate, and colorectal cancer
survivors. Cancer 2009, 115:4001�4009.
195. Bhindi B, Locke J, Alibhai SM, Kulkarni GS, Margel DS, Hamilton RJ, Finelli A,
Trachtenberg J, Zlotta AR, Toi A, Hersey KM, Evans A, van der Kwast TH,
Fleshner NE: Dissecting the association between metabolic syndrome
and prostate cancer risk: analysis of a large clinical cohort. Eur Urol 2014.
doi:10.1016/j.eururo.2014.01.040. [Epub ahead of time]
196. Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Parretta E,
Lenzi A, Giugliano D: Effect of metabolic syndrome and its components
on prostate cancer risk: meta-analysis. J Endocrinol Invest 2013,
36:132�139.
197. U.S. Department of Agriculture and U.S. Department of Health and
Human Services. Dietary Guidelines for Americans, 2010. 7th edition.
Washington, DC: U.S. Government Printing Office, December, 2010.