Why does localized damage or injury caused by trauma lead to chronic, intractable pain in certain patients? What’s in charge of the translation of local injury with acute pain into a chronic pain condition? Why does some pain respond to anti-inflammatory drugs and/or medications, whereas other forms of pain require opiates?
Pain is an intricate process involving both the peripheral nervous system (PNS) and the central nervous system (CNS). Tissue injury triggers the PNS, which transmits signals via the spinal cord into the brain, in which pain perception occurs. However, what causes the intense experience of pain to develop into an unremitting phenomenon? Can anything be done to prevent it? Evidence indicates that chronic pain results from a combination of mechanisms, such as neurological “memories” of preceding pain.
Contents
Nociception: The Simplest Pathway
Acute or nociceptive pain is characterized as the regular experience of discomfort which occurs in response to very basic damage or injury. It is protective, warning us to move away from the origin of the insult and take care of the trauma. The mechanisms that create nociceptive pain include transduction, which extends the external traumatic stimulation into electrical activity in specialized nociceptive primary afferent nerves. The afferent nerves then conduct the sensory information from the PNS to the CNS.
In the CNS, the pain data is transmitted by the primary sensory neurons into central projection cells. After the information is transferred to all those areas of the brain which are responsible for our perception, the actual sensory experience happens. Nociceptive pain is a relatively simple reaction to a particularly simple, acute stimulus. But the mechanics in charge of nociceptive pain cannot identify phenomena, such as pain that persists despite removal or healing of the stimulation, such as in the instance of phantom limb pain.
Pain and the Inflammatory Response
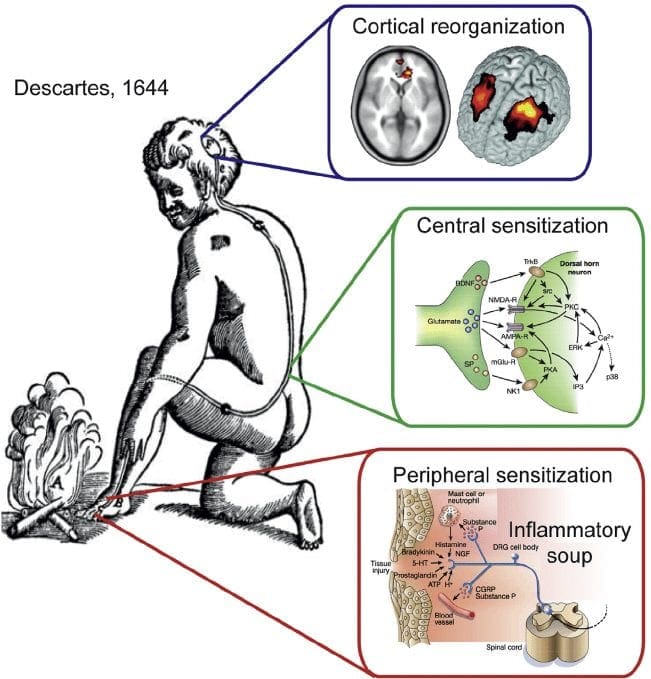
In circumstances of more severe injury, such as surgical wounds, tissue damage may stimulate an inflammatory reaction. However, other conditions, especially arthritis, can also be characterized by continuing cases of inflammation associated with intense pain symptoms. The mechanisms for this type of pain related to tissue damage and an inflammatory response are different from early-warning nociceptive pain.
Observing the incision or site of other damage or injury, a cascade of hyperexcitable events occur in the nervous system. This bodily “wind-up” phenomenon begins at the skin, where it is potentiated along the peripheral nerves, and culminates at a hypersensitivity response along the spinal cord (dorsal horn) and the brain. Inflammatory cells then surround the regions of tissue damage and also produce cytokines and chemokines, substances which are intended to mediate the process of healing and tissue regeneration. But, these agents may also be considered irritants and adjust the properties of the primary sensory neurons surrounding the area of trauma.
Thus, the major factors which trigger inflammatory pain include damage to the high-threshold nociceptors, known as peripheral sensitization, changes and alterations of the neurons in the nervous system, and the amplification of the excitability of neurons within the CNS. This represents central sensitization and is accountable for hypersensitivity, where areas adjacent to those of the true injury will experience pain as if these were injured. These tissues can also react to stimulation which normally doesn’t create pain, such as a touch, wearing clothing, light pressure, or even brushing your own hair, as if they were truly painful, referred to as allodynia.
Peripheral and Central Sensitization (Video)
Other Mechanisms of Pain
Neuropathic pain results from damage or injury to the nervous system, such as carpal tunnel syndrome, postherpetic neuralgia and diabetic neuropathy. Although some of the mechanisms which seem to cause neuropathic pain overlap with those responsible for inflammatory pain, many of them are different, and thus will need a different approach towards their management.
The process of peripheral and central sensitization is maintained, at least theoretically and experimentally, during the excitatory neurotransmitter, glutamate, which is believed to be released when the N-methyl-D-aspartate (NMDA) receptor is activated.
The nervous system is made up of either inhibitory or excitatory neurotransmitters. Most of what permits our nervous system to respond appropriately to damage or injury is the fine-tuning or inhibition of a variety of processes. The overexcitation of the nervous system is seen to be an issue in a number of different disorders. For instance, overactivation of an NMDA receptor can also be related to affective disorders, sympathetic abnormalities, and even opiate tolerance.
Even ordinary nociceptive pain, to some degree, activates the NMDA receptor and is believed to lead to glutamate release. Nonetheless, in neuropathic pain, oversensitivity to the NMDA receptor is key.
With other types of chronic pain, such as fibromyalgia and tension-type headaches, some of the mechanisms active in inflammatory and neuropathic pain may also create similar abnormalities in the pain system, including central sensitization, higher excitability of the somatosensory pathways, and reductions in central nervous system inhibitory mechanisms.
Peripheral Sensitization
Cyclo-oxygenase (COX) also plays an essential function in both peripheral and central sensitizations. COX-2 is one of the enzymes which are induced during the inflammatory process; COX-2 converts arachidonic acid into prostaglandins, which increase the sensitivity of peripheral nociceptor terminals. Virtually, peripheral inflammation also causes COX-2 to be produced from the CNS. Signals from peripheral nociceptors are partially responsible for this upregulation, but there also seems to be a humoral component to the transduction of the pain signals across the blood-brain barrier.
For instance, in experimental models, COX-2 is generated from the CNS even if animals receive a sensory nerve block prior to peripheral inflammatory stimulation. The COX-2 that is expressed over the dorsal horn neurons of the spinal cord releases prostaglandins, which act on the central terminals, or the presynaptic terminals of nociceptive sensory fibers, to increase transmitter release. Additionally, they act postsynaptically on the dorsal horn neurons to produce direct depolarization. And finally, they inhibit the activity of glycine receptor, and this is an inhibitory transmitter. Therefore, the prostaglandins create an increase in excitability of central neurons.
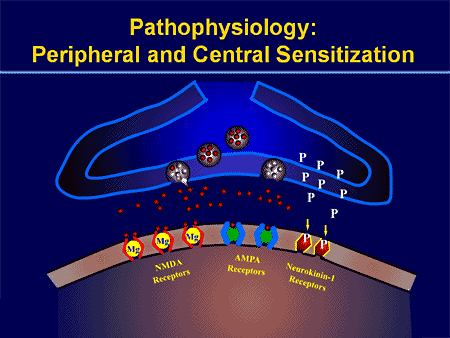
Brain Plasticity and Central Sensitization
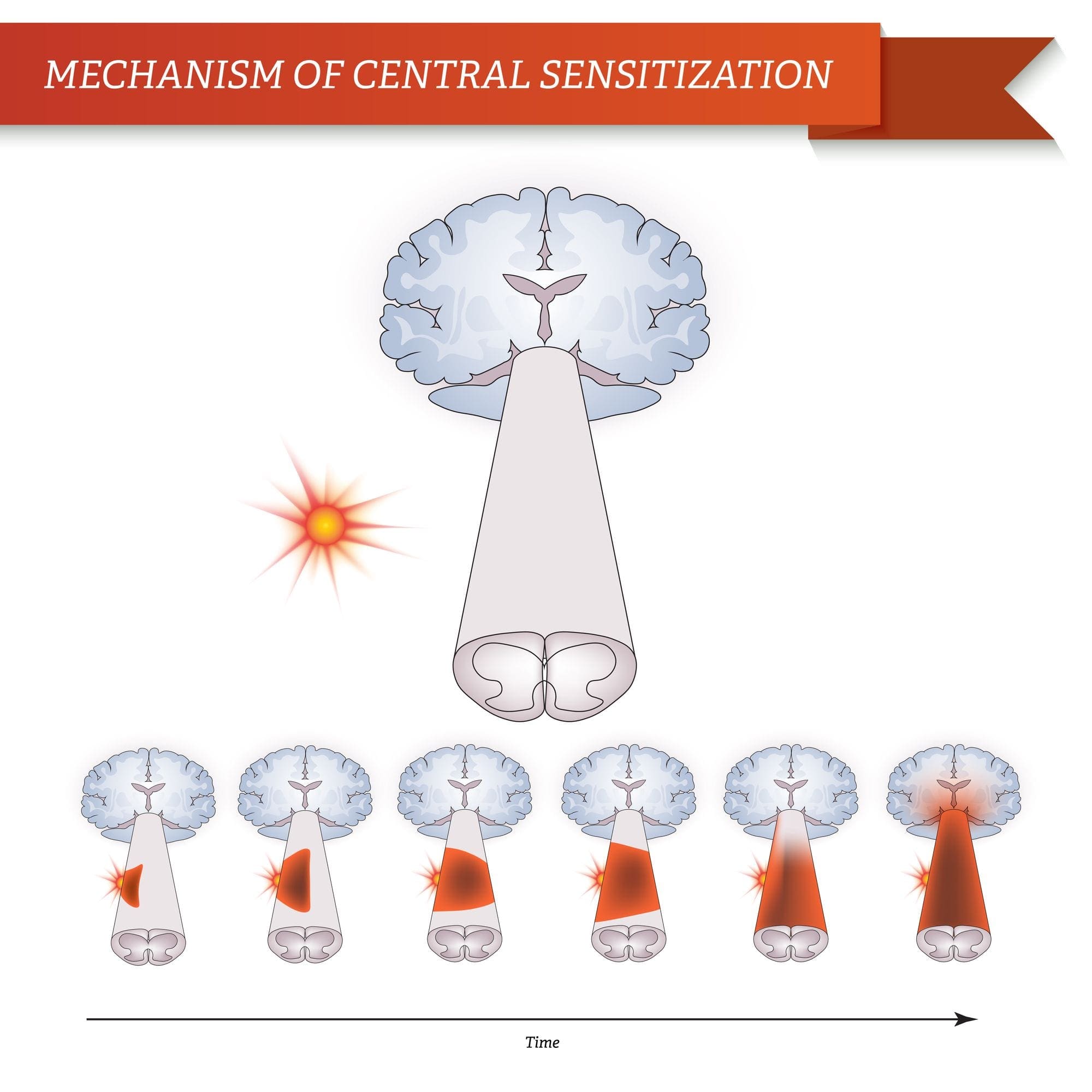
Central sensitization describes changes which happen in the brain in reaction to repeated nerve stimulation. After repeated stimuli, amounts of hormones and brain electric signals change as neurons develop a “memory’ for reacting to those signs. Constant stimulation creates a more powerful brain memory, so the brain will respond more rapidly and effectively when undergoing the identical stimulation in the future. The consequent modifications in brain wiring and reaction are referred to as neural plasticity, which describe the capability of the brain to alter itself readily, or central sensitization. Therefore, the brain is activated or sensitized by previous or repeated stimuli to become more excitable.
The fluctuations of central sensitization occur after repeated encounters with pain. Research in animals indicates that repeated exposure to a painful stimulation will change the animal’s pain threshold and lead to a stronger pain response. Researchers think that these modifications can explain the persistent pain that could occur even after successful back surgery. Although a herniated disc may be removed from a pinched nerve, pain may continue as a memory of the nerve compression. Newborns undergoing circumcision without anesthesia will react more profoundly to future painful stimulation, such as routine injections, vaccinations, and other painful processes. These children haven’t only a higher hemodynamic reaction, known as tachycardia and tachypnea, but they will also develop enhanced crying too.
This neurological memory of pain was studied extensively. In a report on his previous research studies, Woolf noted that the improved reflex excitability following peripheral tissue damage or injury doesn’t rely on continuing peripheral input signals; rather, hours after a peripheral trauma, spinal dorsal horn neuron receptive fields continued to enlarge. Researchers also have documented the significance of the spinal NMDA receptor to the induction and maintenance of central sensitization.
Significance for Pain Management
Once central sensitization is established, bigger doses of analgesics are often required to suppress it. Preemptive analgesia, or therapy before pain progresses, may lower the effects of all of these stimulation on the CNS. Woolf demonstrated that the morphine dose required to stop central hyperexcitability, given before short noxious electrical stimulation in rats, was one tenth the dose required to abolish activity after it had grown. This translates to clinical practice.
In a clinical trial of 60 patients undergoing abdominal hysterectomy, individuals who received 10 mg of morphine intravenously at the time of induction of anesthesia required significantly less morphine for postoperative pain control. Furthermore, pain sensitivity around the wound, referred to as secondary hyperalgesia, was also reduced in the morphine pretreated group. Preemptive analgesia was used with comparable success in an assortment of surgical settings, including prespinal operation and postorthopaedic operation.
A single dose of 40 or 60 mg/kg of rectal acetaminophen has a clear morphine-sparing effect in day-case surgery in children, if administered in the induction of anesthesia. Furthermore, children with sufficient analgesia with acetaminophen experienced significantly less postoperative nausea and vomiting.
NMDA receptor antagonists have imparted postoperative analgesia when administered preoperatively. Various reports exist in the literature supporting the use of ketamine and dextromethorphan in the preoperative period. In patients undergoing anterior cruciate ligament reconstruction, 24-hour patient-controlled analgesia opioid consumption was significantly less in the preoperative dextromethorphan category versus the placebo group.
In double-blind, placebo-controlled research studies, gabapentin was indicated as a premedicant analgesic for patients undergoing mastectomy and hysterectomy. Preoperative oral gabapentin reduced pain scores and postoperative analgesic consumption without gap in side effects as compared with placebo.
Preoperative administration of nonsteroidal anti-inflammatory drugs (NSAIDs) has demonstrated a significant decrease in opioid use postoperatively. COX-2s are preferable due to their relative lack of platelet effects and significant gastrointestinal safety profile when compared with conventional NSAIDs. Celecoxib, rofecoxib, valdecoxib, and parecoxib, outside the United States, administered preoperatively reduce postoperative narcotic use by more than 40 percent, with many patients using less than half of the opioids compared with placebo.
Blocking nerve conduction in the preoperative period appears to prevent the development of central sensitization. Phantom limb syndrome (PLS) has been attributed to a spinal wind-up phenomenon.�Patients with amputation
often have burning or tingling pain in the body part removed. One possible cause is that nerve fibers at the stump are stimulated and the brain interprets the signals as originating in the amputated portion. The other is the rearrangement within the cortical areas so that area say for the hand now responds to signals from other parts of the body but still interprets them as coming for the amputated hand.
However, for patients undergoing lower-extremity amputation under epidural anesthesia, not one of the 11 patients who received lumbar epidural blockade with bupivacaine and morphine for 72 hours before operation developed PLS. For people who underwent general anesthesia without prior lumbar epidural blockade, 5 of 14 patients had PLS at 6 weeks and 3 continued to experience PLS at 1 year.
Woolf and Chong have noted that perfect preoperative, intraoperative, and postoperative treatment comprises of “NSAIDs to reduce the activation/centralization of nociceptors, local anesthetics to block sensory inflow, and centrally acting drugs such as opiates.” Decreasing perioperative pain with preemptive techniques enhances satisfaction, hastens discharge, spares opioid use, along with diminished constipation, sedation, nausea, and urinary retention, and may even stop the development of chronic pain. Anesthesiologists and surgeons should consider integrating these techniques in their everyday practices.
When pain occurs as a result of damage or injury in consequence of surgery, the spinal cord can attain a hyperexcitable state wherein excessive pain reactions occur that may persist for days, weeks or even years.
Why does localized injury resulting from trauma result in chronic, intractable pain in some patients? Tissue injury leads to a constellation of changes in spinal excitability, including elevated spontaneous firing, greater response amplitude and length, decreased threshold, enhanced discharge to repeated stimulation, and expanded receptive fields. The persistence of these changes, which are collectively termed central sensitization, appears to be fundamental to the prolonged enhancement of pain sensitivity which defines chronic pain. Numerous drugs and/or medications as well as local anesthetic neural blockade may limit the magnitude of the central nervous system (CNS) windup, as evidenced by diminished pain and diminished opioid consumption in the preemptive analgesic models.

Dr. Alex Jimenez’s Insight
Chiropractic care is an alternate treatment option which utilizes spinal adjustments and manual manipulations to safely and effectively restore as well as maintain the proper alignment of the spine. Research studies have determined that spinal misalignments, or subluxations, can lead to chronic pain. Chiropractic care is commonly utilized for pain management, even if the symptoms are not associated to an injury and/or condition in the musculoskeletal and nervous system. By carefully re-aligning the spine, a chiropractor can help reduce stress and pressure from the structures surrounding the main component of out body’s foundation, ultimately providing pain relief.
Enteric Nervous System Function and Pain
When it comes to the diminished use of drugs and/or medications, including opioids, in order to prevent side-effects like gastrointestinal health issues, the proper function of the enteric nervous system may be at play.
The enteric nervous system (ENS) or intrinsic nervous system is one of the key branches of the autonomic nervous system (ANS) and consists of a mesh-like system of nerves which modulates the role of the gastrointestinal tract. It’s capable of acting independently of the sympathetic and parasympathetic nervous systems, even though it might be affected by them. The ENS can also be called the second brain.�It is derived from neural crest cells.
The enteric nervous system in humans is made up of some 500 million neurons, including the numerous types of Dogiel cells, approximately one two-hundredth of the amount of neurons in the brain. The enteric nervous system is inserted into the lining of the gastrointestinal system, beginning at the esophagus and extending down to the anus. Dogiel cells, also known as cells of Dogiel, refers to some kind of multipolar adrenal tissues within the prevertebral sympathetic ganglia.
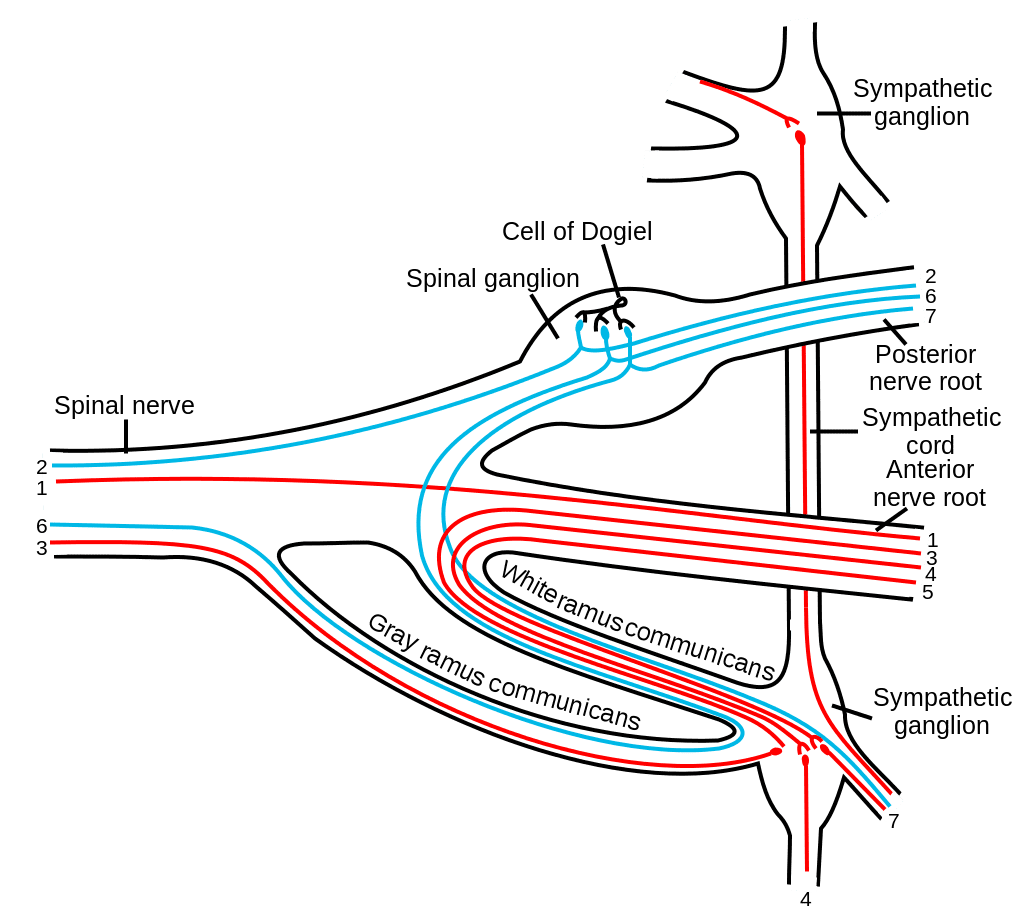
The ENS is capable of autonomous functions, such as the coordination of reflexes; even though it receives considerable innervation in the autonomic nervous system, it does and can operate independently of the brain and the spinal cord.�The enteric nervous system has been described as the “second brain” for a number of reasons. The enteric nervous system may operate autonomously. It normally communicates with the central nervous system (CNS) via the parasympathetic, or via the vagus nerve, and the sympathetic, that is through the prevertebral ganglia, nervous systems. However, vertebrate studies reveal that when the vagus nerve is severed, the enteric nervous system continues to function.
In vertebrates, the enteric nervous system includes efferent neurons, afferent neurons, and interneurons, all of which make the enteric nervous system capable of carrying reflexes and acting as an integrating center in the absence of CNS input. The sensory neurons report on mechanical and chemical conditions. The enteric nervous system has the ability to change its response based on such factors as nutrient and bulk composition. In addition, ENS contains support cells that are much like astroglia of the brain and a diffusion barrier around the capillaries surrounding ganglia that’s like the blood-brain barrier of blood vessels.
The enteric nervous system (ENS) plays a pivotal role in inflammatory and nociceptive processes. Drugs and/or medications that interact with the ENS have recently raised considerable interest because of their capacity to regulate numerous aspects of the gut physiology and pathophysiology. In particular, experiments in animals have demonstrated that�proteinase-activated receptors (PARs) may be essential to neurogenic inflammation in the intestine. Moreover, PAR2 agonists seem to induce intestinal hypersensitivity and hyperalgesic states, suggesting a role for this receptor in visceral pain perception.
Furthermore, PARs, together with the proteinases that activate them, represent exciting new targets for therapeutic intervention on the ENS. The scope of our information is limited to chiropractic as well as to spinal injuries and conditions. To discuss the subject matter, please feel free to ask Dr. Jimenez or contact us at 915-850-0900 .
Curated by Dr. Alex Jimenez
Additional Topics: Sciatica
Sciatica is medically referred to as a collection of symptoms, rather than a single injury and/or condition. Symptoms of sciatic nerve pain, or sciatica, can vary in frequency and intensity, however, it is most commonly described as a sudden, sharp (knife-like) or electrical pain that radiates from the low back down the buttocks, hips, thighs and legs into the foot. Other symptoms of sciatica may include, tingling or burning sensations, numbness and weakness along the length of the sciatic nerve. Sciatica most frequently affects individuals between the ages of 30 and 50 years. It may often develop as a result of the degeneration of the spine due to age, however, the compression and irritation of the sciatic nerve caused by a bulging or herniated disc, among other spinal health issues, may also cause sciatic nerve pain.

EXTRA IMPORTANT TOPIC: Chiropractor Sciatica Symptoms
MORE TOPICS: EXTRA EXTRA: El Paso Back Clinic | Back Pain Care & Treatments
General Disclaimer, Licenses and Board Certifications *
Professional Scope of Practice *
The information herein on "Understanding Abnormalities of the Pain System in El Paso, Tx" is not intended to replace a one-on-one relationship with a qualified health care professional or licensed physician and is not medical advice. We encourage you to make healthcare decisions based on your research and partnership with a qualified healthcare professional.
Blog Information & Scope Discussions
Welcome to El Paso's Premier Wellness and Injury Care Clinic & Wellness Blog, where Dr. Alex Jimenez, DC, FNP-C, a Multi-State board-certified Family Practice Nurse Practitioner (FNP-BC) and Chiropractor (DC), presents insights on how our multidisciplinary team is dedicated to holistic healing and personalized care. Our practice aligns with evidence-based treatment protocols inspired by integrative medicine principles, similar to those on this site and on our family practice-based chiromed.com site, focusing on naturally restoring health for patients of all ages.
Our areas of multidisciplinary practice include Wellness & Nutrition, Chronic Pain, Personal Injury, Auto Accident Care, Work Injuries, Back Injury, Low Back Pain, Neck Pain, Migraine Headaches, Sports Injuries, Severe Sciatica, Scoliosis, Complex Herniated Discs, Fibromyalgia, Complex Injuries, Stress Management, Functional Medicine Treatments, and in-scope care protocols.
Our information scope is multidisciplinary, focusing on musculoskeletal and physical medicine, wellness, contributing etiological viscerosomatic disturbances within clinical presentations, associated somato-visceral reflex clinical dynamics, subluxation complexes, sensitive health issues, and functional medicine articles, topics, and discussions.
We provide and present clinical collaboration with specialists from various disciplines. Each specialist is governed by their professional scope of practice and their jurisdiction of licensure. We use functional health & wellness protocols to treat and support care for musculoskeletal injuries or disorders.
Our videos, posts, topics, and insights address clinical matters and issues that are directly or indirectly related to our clinical scope of practice.
Our office has made a reasonable effort to provide supportive citations and has identified relevant research studies that support our posts. We provide copies of supporting research studies upon request to regulatory boards and the public.
We understand that we cover matters that require an additional explanation of how they may assist in a particular care plan or treatment protocol; therefore, to discuss the subject matter above further, please feel free to ask Dr. Alex Jimenez, DC, APRN, FNP-BC, or contact us at 915-850-0900.
We are here to help you and your family.
Blessings
Dr. Alex Jimenez, DC, MSACP, APRN, FNP-BC*, CCST, IFMCP, CFMP, ATN
email: [email protected]
Multidisciplinary Licensing & Board Certifications:
Licensed as a Doctor of Chiropractic (DC) in Texas & New Mexico*
Texas DC License #: TX5807, Verified: TX5807
New Mexico DC License #: NM-DC2182, Verified: NM-DC2182
Multi-State Advanced Practice Registered Nurse (APRN*) in Texas & Multi-States
Multi-state Compact APRN License by Endorsement (42 States)
Texas APRN License #: 1191402, Verified: 1191402 *
Florida APRN License #: 11043890, Verified: APRN11043890 *
Colorado License #: C-APN.0105610-C-NP, Verified: C-APN.0105610-C-NP
New York License #: N25929, Verified N25929
License Verification Link: Nursys License Verifier
* Prescriptive Authority Authorized
ANCC FNP-BC: Board Certified Nurse Practitioner*
Compact Status: Multi-State License: Authorized to Practice in 40 States*
Graduate with Honors: ICHS: MSN-FNP (Family Nurse Practitioner Program)
Degree Granted. Master's in Family Practice MSN Diploma (Cum Laude)
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card
Licenses and Board Certifications:
DC: Doctor of Chiropractic
APRNP: Advanced Practice Registered Nurse
FNP-BC: Family Practice Specialization (Multi-State Board Certified)
RN: Registered Nurse (Multi-State Compact License)
CFMP: Certified Functional Medicine Provider
MSN-FNP: Master of Science in Family Practice Medicine
MSACP: Master of Science in Advanced Clinical Practice
IFMCP: Institute of Functional Medicine
CCST: Certified Chiropractic Spinal Trauma
ATN: Advanced Translational Neutrogenomics
Memberships & Associations:
TCA: Texas Chiropractic Association: Member ID: 104311
AANP: American Association of Nurse Practitioners: Member ID: 2198960
ANA: American Nurse Association: Member ID: 06458222 (District TX01)
TNA: Texas Nurse Association: Member ID: 06458222
NPI: 1205907805
| Primary Taxonomy | Selected Taxonomy | State | License Number |
|---|---|---|---|
| No | 111N00000X - Chiropractor | NM | DC2182 |
| Yes | 111N00000X - Chiropractor | TX | DC5807 |
| Yes | 363LF0000X - Nurse Practitioner - Family | TX | 1191402 |
| Yes | 363LF0000X - Nurse Practitioner - Family | FL | 11043890 |
| Yes | 363LF0000X - Nurse Practitioner - Family | CO | C-APN.0105610-C-NP |
| Yes | 363LF0000X - Nurse Practitioner - Family | NY | N25929 |
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card