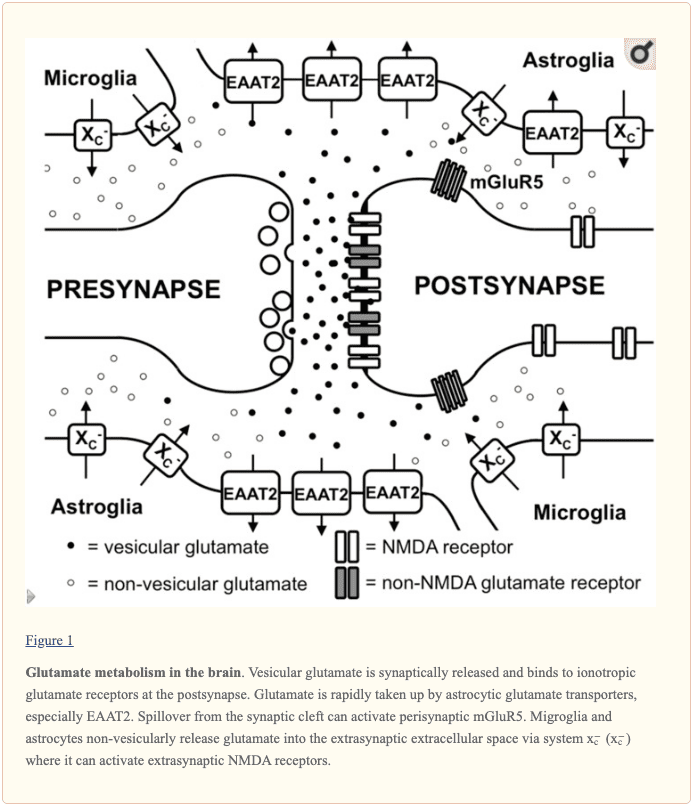
Functional Neurology: Other Molecules in Glutamate
Previous research studies suggest that L-aspartate, like L-glutamate, triggers excitatory activity on neurons. L-aspartate functions with L-glutamate in the synaptic vesicles of asymmetric excitatory synapses. But, the total concentration of these in the human brain (0.96-1.62 ?mol/gram wet weight), their extracellular concentrations in the cortex as measured by microdialysis (1.62 ?M for L-aspartate and 9.06 ?M for L-glutamate) and their supply according to immunohistochemistry suggest that L-aspartate is significantly less abundant than L-glutamate. Moreover, L-aspartate is a powerful agonist for NMDA receptors but not for other iGluRs with an EC50 just eight-fold higher than that of L-glutamate. EAATs which play a fundamental role in the uptake of all vesicular released L-glutamate in the central nervous system (CNS) also requires the utilization of L-aspartate. L-aspartate is perhaps as less essential as L-glutamate connected to the total excitatory activity associated with iGluRs. Along with its role as a neurotransmitter, as previously mentioned, L-aspartate is also necessary as a substrate for aspartate amino-transferase which turns into 2-oxoglutarate and L-glutamate to transport to the cortical vesicles of glutamatergic neurons which may also consequently and indirectly increase L-glutamate release. �
Other Molecules in Glutamate Signaling
One characteristic which distinguishes NMDA receptors from different iGluRs is that the activation of NMDA receptors needs the connection of a co-agonist to the glycine binding region of the receptor. By way of instance, in the retina and in the spinal cord, the origin of glycine may spillover out of glycinergic inhibitory synapses. But, in different regions of the brain with increased NMDA receptor expression, such as the hippocampal formation, reactions associated with strychnine-sensitive glycine receptors are missing, at least in adult neurons, demonstrating the absence of glycinergic inhibitory neurotransmissions. But, glycine is found in the extracellular fluid of the hippocampus at baseline amounts of roughly 1.5 ?M, which is similar to the saturation of the glycine binding region of the NMDA receptor, although these may be up- and down-regulated. The origin of extracellular glycine in the hippocampus can be neurons which release glycine through the alanine-serine-cysteine amino acid transporter 1 (asc-1). But, glycine release by astrocytes that is stimulated by depolarization and kainate, has also been demonstrated. Further research studies are required to ultimately show these outcome measures. �
Even in previous research studies of the NMDA receptor and its co-activation by glycine revealed that D-amino acids, particularly D-serine, are nearly as powerful as glycine. Only several years after, it became obvious that D-serine is found in rat and human brains at roughly one-third of their concentration of L-serine having an absolute concentration of more than 0.2 ?mol/g brain tissue. Utilizing an antiserum for D-serine, research studies demonstrated that D-serine from the brain is only found in astrocytes and its supply fits the expression of NMDA receptors. In addition, the same researchers demonstrated that D-serine is released from cultured astrocytes when exposed to L-glutamate or kainate. The abundance of D-serine is found by the degrading enzyme D-amino acid oxidase (DAO) which reveals increased expression in the hindbrain where D-serine levels are reduced as well as the synthetic enzyme serine racemase which creates D-serine from L-serine. D-Serine appears to be stored in cytoplasmic vesicles in astrocytes and it can be released by exocytosis. Long-term potentiation is dependent upon D-serine release from astrocytes in hippocampal slices, suggesting that this amino acid definitely plays a fundamental role in glutamatergic neurotransmission through NMDA receptors. Additionally in hippocampal slices, research studies found, utilizing D-serine and glycine degrading enzymes, which D-serine functions as a co-transmitter for synaptic NMDA receptors on CA1 neurons likewise which glycine functions as the endogenous co-agonist for extrasynaptic NMDA receptors. Synaptic NMDA receptors of dentate gyrus neurons utilize glycine rather than D-serine as the co-agonist. �
Taken collectively, multilayered outcome measures show that L-aspartate doesn’t simply function as an agonist on NMDA receptors but also glycine and D-serine play fundamental roles in glutamatergic neurotransmission in the human brain. But, other molecules also have been demonstrated to be relevant modulators of glutamatergic neurotransmission. �
Glutamate Activated by Other Molecules
L-homocysteate (L-HCA) has structural similarities with L-glutamate. The non-protein amino acid is an oxidation product of homocysteine that is biosynthesized from methionine in the elimination of its own terminal methyl group and it is also an intermediate of the transsulfuration pathway by which methionine may be converted to cysteine through cystathionine. Early research studies demonstrated that this amino acid can cause calcium influx in cultured neurons as safely and effectively as L-glutamate. Moreover, L-HCA revealed an increased affinity for NMDA receptors when compared to other iGluRs in binding assays associated with its capacity to cause NMDA receptor antagonist-inhibitable excitotoxicity and sodium influx. Additionally, L-HCA can trigger mGluR5 as efficiently as L-glutamate. L-HCA is found in the brain, however, the concentrations were demonstrated to be approximately 500-fold lesser than those of L-glutamate and even 100-fold lesser when compared to those of L-aspartate in different regions of the rat brain. Throughout potassium-induced stimulation, L-HCA discharge is triggered from brain slice preparations as demonstrated for L-aspartate and L-glutamate although the absolute release of HCA is approximately 50-fold lesser. Surprisingly, HCA is a very efficient competitive inhibitor of cystine and L-glutamate uptake through the cystine/glutamate antiporter system x?c, the activity that regulates and manages the extracellular extrasynaptic L-glutamate concentrations in the brain. Therefore, the impact of L-HCA on the activation of NMDA and other L-glutamate receptors may also rely on the L-HCA-induced trigger of L-glutamate through system x?c. L-HCA may play an important role in the overall stimulation of L-glutamate receptors. Nevertheless, this can change tremendously under certain conditions, e.g., in patients with high-dose methotrexate therapy, an anticancer drug which, by restricting dihydrofolate reductase, limits the tetrahydrofolate-catalyzed recycling of methionine from homocysteine. Here, L-HCA concentrations of more than 100 ?M have been demonstrated from the cerebrospinal fluid whereas L-HCA was undetectable in control subjects. Further research studies are still required to determine these outcome measures. �
Further endogenous small molecules which are believed to affect L-glutamate signaling include several intermediates of tryptophan metabolism, as shown in Figure 2. Through the activity of indoleamine 2,3-dioxygenase (IDO) or tryptophan 2,3-dioxygenase (TDO), tryptophan is turned into N-formyl-L-kynurenine which is later turned into kynurenine (KYN) by formamidase. Three pathways, two of which connect at a subsequent step, result in further metabolism. First, through the activity of kynurenine aminotransferase (KAT), KYN is converted into kynurenic acid (KYNA). KYN can also be converted to 3-hydroxykynurenine (3HK) by kynurenine monooxygenase (KMO), which can subsequently be utilized as a substrate by kynureninase for the synthesis of 3-hydroxyanthranilic acid (3HANA). Additionally, utilizing KYN as a substrate, kynureninase develops anthranilic acid (ANA), which by non-specific hydroxylation may also be converted to 3HANA. According to research studies, 3HANA finally functions as a substrate for the generation of quinolinic acid (QUIN). �
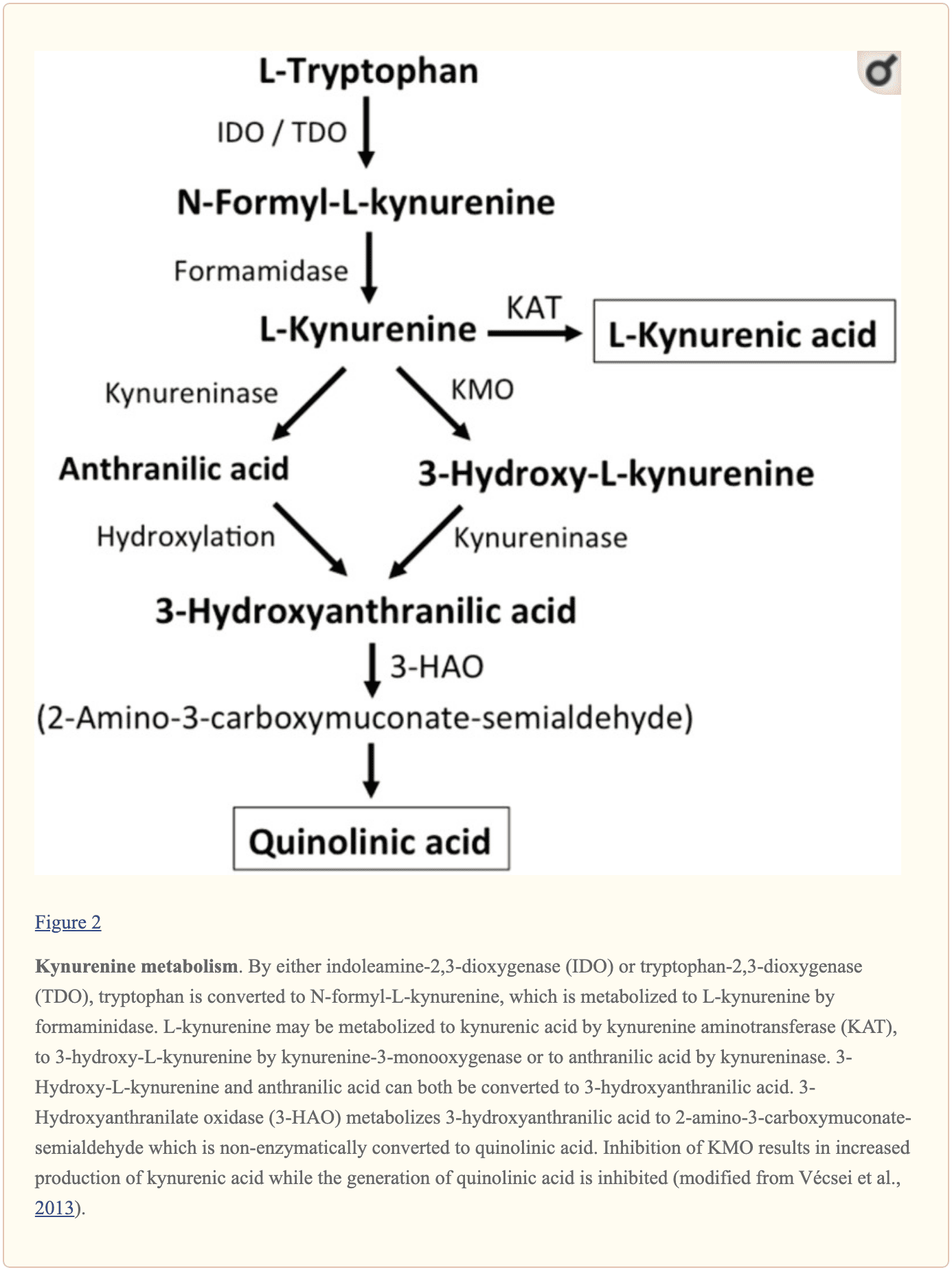
The tryptophan concentration in the rat brain is roughly 25 nmol/g wet weight and approximately 400-fold less than L-glutamate and 100-fold less than L-aspartate. The demonstrated brain levels of kynurenines are even lower with 0.4-1.6 nmol/g for QUIN, 0.01-0.07 nmol/ml for KYNA, and 0.016 nmol/g for 3HANA. Approximately 40 percent of brain KYN is locally synthesized. The metabolites of tryptophan demonstrate differential binding to plasma proteins and their transport through the barrier which is quite different. KYN and 3HK are carried through the large neutral amino acid carrier system L. Kynurenines seem to penetrate the human brain by passive diffusion. Additionally, KYNA, 3HANA, and especially ANA bind to serum proteins which then ultimately restrict and limit their diffusibility across the blood-brain barrier. �
Research studies demonstrated that QUIN, when ionophoretically utilized in rat cells, caused neuronal firing which has been prevented by an NMDA receptor antagonist, suggesting that QUIN may function as an NMDA receptor agonist. However, the EC50 for QUIN to trigger NMDA receptor currents has been shown to be roughly 1000-fold higher than the EC50 of L-glutamate. Intracerebral injection of QUIN was proven to cause ultrastructural, neurochemical, and behavioral changes similar to those caused by NMDA receptor agonists. The fact that QUIN concentrations are about 5000- to 15,000-fold lower than cerebral L-glutamate concentrations makes it unlikely that modulation of NMDA receptor signaling by QUIN plays an essential role. KYNA was demonstrated to function as an NMDA receptor antagonist. But, although infusion with the KMO inhibitor Ro 61-8048 improved cerebral extracellular KYNA concentrations 10-fold, this didn’t result in an inhibition of NMDA-mediated neuronal depolarization, a finding which challenges the belief that KYNA at near-physiological amounts directly modulates NMDA receptors. In comparison, increased KYNA in the brain induced from the KMO inhibitor JM6 decreased the extracellular cerebral L-glutamate concentration. Additionally, KYNA levels from the extracellular cerebral fluid have been associated with L-glutamate levels suggesting that even at physiological or near physiological levels, KYNA modulates L-glutamate metabolism. Both the activation of the G-protein-coupled receptor GPR35 and the inhibition of presynaptic ?7 nicotinic acetylcholine receptors are suggested in the KYNA-induced reduction in L-glutamate release. To summarize, although QUIN and L-HCA are present in the human brain, their concentrations discuss against them with roles in regulating and maintaining neurotransmission. In contrast, even though the pathways have to be defined in greater detail, evidence supports levels and the opinion that discharge can be modulated by KYNA and neurotransmission. �

Glutamate, together with aspartate and other molecules, are several of the main excitatory neurotransmitters in the human brain. Although these play a fundamental role in the overall structure and function of the central nervous system, including the brain and the spinal cord, excessive amounts of other molecules can ultimately trigger glutamate receptors. Excess glutamate can cause excitotoxicity which may lead to a variety of health issues, such as Alzheimer’s disease and other types of neurological diseases. The following article describes how other molecules can activate glutamate receptors. – Dr. Alex Jimenez D.C., C.C.S.T. Insight – Dr. Alex Jimenez D.C., C.C.S.T. Insight
Research studies suggest that L-aspartate, like L-glutamate, triggers excitatory activity. L-aspartate functions with L-glutamate in the synaptic vesicles of asymmetric excitatory synapses. But, the total concentration of these in the human brain suggest that L-aspartate is significantly less abundant than L-glutamate. Moreover, L-aspartate is a powerful agonist for NMDA receptors but not for other iGluRs with an EC50 just eight-fold higher than that of L-glutamate. The scope of our information is limited to chiropractic, musculoskeletal and nervous health issues as well as functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or chronic disorders of the musculoskeletal system. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900 . �
Curated by Dr. Alex Jimenez �
References �
- Lewerenz, Jan, and Pamela Maher. �Chronic Glutamate Toxicity in Neurodegenerative Diseases-What Is the Evidence?� Frontiers in Neuroscience, Frontiers Media S.A., 16 Dec. 2015, www.ncbi.nlm.nih.gov/pmc/articles/PMC4679930/.
Additional Topic Discussion: Chronic Pain
Sudden pain is a natural response of the nervous system which helps to demonstrate possible injury. By way of instance, pain signals travel from an injured region through the nerves and spinal cord to the brain. Pain is generally less severe as the injury heals, however, chronic pain is different than the average type of pain. With chronic pain, the human body will continue sending pain signals to the brain, regardless if the injury has healed. Chronic pain can last for several weeks to even several years. Chronic pain can tremendously affect a patient’s mobility and it can reduce flexibility, strength, and endurance.
Neural Zoomer Plus for Neurological Disease
Dr. Alex Jimenez utilizes a series of tests to help evaluate neurological diseases. The Neural ZoomerTM Plus is an array of neurological autoantibodies which offers specific antibody-to-antigen recognition. The Vibrant Neural ZoomerTM Plus is designed to assess an individual�s reactivity to 48 neurological antigens with connections to a variety of neurologically related diseases. The Vibrant Neural ZoomerTM Plus aims to reduce neurological conditions by empowering patients and physicians with a vital resource for early risk detection and an enhanced focus on personalized primary prevention. �
Formulas for Methylation Support
XYMOGEN�s Exclusive Professional Formulas are available through select licensed health care professionals. The internet sale and discounting of XYMOGEN formulas are strictly prohibited
Proudly,�Dr. Alexander Jimenez makes XYMOGEN formulas available only to patients under our care.
Please call our office in order for us to assign a doctor consultation for immediate access.
If you are a patient of Injury Medical & Chiropractic�Clinic, you may inquire about XYMOGEN by calling 915-850-0900.
For your convenience and review of the XYMOGEN products please review the following link.*XYMOGEN-Catalog-Download �
* All of the above XYMOGEN policies remain strictly in force.
�