Biomarkers (short for biological markers) are biological measurements of a biological condition. By definition, a biomarker is “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention” Biomarkers are the measurements utilized to perform a clinical evaluation like blood pressure or cholesterol level and therefore are used to monitor and forecast health conditions in individuals or across populations so that appropriate treatment options could be proposed. Biomarkers may be used by itself or in combination to assess the health or disease state of an individual.
Contents
Variety of Biomarkers
A wide selection of biomarkers are used now. Every biological system, such as the cardiovascular system, metabolic system or the immune system, has its own specific biomarkers. Many of these biomarkers are rather easy to quantify and form part of regular medical examinations. By way of instance, a general health check may include assessment of blood pressure, heart rate, cholesterol, triglycerides and fasting glucose levels. Body dimensions such as weight, body mass index, or BMI, and waist-to-hip ratio are routinely used for assessing conditions like obesity and metabolic disorders, among others. These varieties in biomarkers can ultimately be useful in the diagnosis of a variety of health issues.
Attributes of a Perfect Biomarker
An ideal biomarker has particular characteristics that make it suitable for assessing a particular disease or condition. Ideally, an ideal marker should possess the following features, as it should be:
- Safe and simple to measure
- Cost effective to follow up
- Modifiable with treatment
- Consistent across gender and cultural groups
Biomarkers as Health and Disease Predictors
Biomarkers are used to predict significant ailments like diabetes and cardiovascular disease, among others. Each individual biomarker indicates whether there’s a disease or health condition and can be combined to offer a thorough demonstration of how healthy an individual is and whether further diagnosis needs to be made. Biomarkers ultimately serve as health and disease predictors, capable of determining a potential onset of disease or illness, such as that of cancer.
Biomarkers in Cancer Detection and Drug Development
The principles of biomarkers in disease have been applied to the discovery, screening, diagnosis, treatment and monitoring of cancer. Traditionally, anti-cancer drugs and/or medications were agents that eliminated both cancer cells and healthy cells. However, more targeted therapies have now been developed that can be instructed to kill cancer cells only, while sparing healthy cells. The evaluation of a typical biomarker in cancer will help in the development of therapies that may target the biomarker. This can minimize the risk of toxicity and reduce the cost of treatment. In cancer research, genetic studies are valuable because genetic abnormalities so often underlie the evolution of cancer. Certain DNA or RNA markers might therefore help in the treatment and detection of specific cancers. The purpose of the following article, however, is to demonstrate the biomarkers involved in low back pain, disc degeneration and other chronic pain health issues, such as neuropathic pain.
Inflammatory Biomarkers of Low Back Pain and Disc Degeneration: a Review
Abstract
Biomarkers are biological characteristics that can be used to indicate health or disease. This paper reviews studies on biomarkers of low back pain (LBP) in human subjects. LBP is the leading cause of disability, caused by various spine-related disorders, including intervertebral disc degeneration, disc herniation, spinal stenosis, and facet arthritis. The focus of these studies is inflammatory mediators, because inflammation contributes to the pathogenesis of disc degeneration and associated pain mechanisms. Increasingly, studies suggest that the presence of inflammatory mediators can be measured systemically in the blood. These biomarkers may serve as novel tools for directing patient care. Currently, patient response to treatment is unpredictable with a significant rate of recurrence, and, while surgical treatments may provide anatomical correction and pain relief, they are invasive and costly. The review covers studies performed on populations with specific diagnoses and undefined origins of LBP. Since the natural history of LBP is progressive, the temporal nature of studies is categorized by duration of symptomology/disease. Related studies on changes in biomarkers with treatment are also reviewed. Ultimately, diagnostic biomarkers of LBP and spinal degeneration have the potential to shepherd an era of individualized spine medicine for personalized therapeutics in the treatment of LBP.
Keywords: back pain; biomarkers; inflammation; intervertebral disc degeneration; spine
Biomarkers for Chronic Neuropathic Pain and their Potential Application in Spinal Cord Stimulation: a Review
Abstract
This review was focused on understanding which substances inside the human body increase and decrease with increasing neuropathic pain. We reviewed various studies, and saw correlations between neuropathic pain and components of the immune system (this system defends the body against diseases and infections). Our findings will especially be useful for understanding ways to reduce or eliminate the discomfort, chronic neuropathic pain brings with it. Spinal cord stimulation (SCS) procedure is one of the few fairly efficient remedial treatments for pain. A follow-up study will apply our findings from this review to SCS, in order to understand the mechanism, and further optimize efficaciousness.
Keywords: spinal cord stimulation, pain biomarkers, chronic neuropathic pain, cytokines
Introduction
Chronic neuropathic pain disorders represent a common long-term disability in the United States, as well as, globally. They affect 1 in 4 Americans. Chronic neuropathic pain treatment has limited success because of poor understanding of the mechanisms that lead to the initiation and maintenance. Additionally, the effectiveness of neuropathic pain management regimens and procedures has been difficult to determine in the past, due to the subjectivity related to pain perception, and a lack of standardized assessment of neuropathic pain. However, one of the most effective management strategies in recent times is spinal cord stimulation (SCS). The main goals of spinal cord stimulation are to improve physical function and quality of life in the patients afflicted with chronic neuropathic pain associated with complex regional pain syndrome (CRPS), failed back syndrome, and other chronic neuropathic pain syndromes [1�2]. Despite limited knowledge of how people benefit from SCS, more than 20,000 stimulators are implanted each year, with more than a half-billion dollars in revenue [3]. While it is generally believed that spinal cord stimulation inhibits pain transmission in the dorsal horn, the exact mechanisms by which SCS relieves neuropathic pain is unknown. Chronic neuropathic pain is caused often by inflammation and/or nerve injury. The advances have shown that inflammation and nerve injury produce changes in the expression of cytokines, neurotransmitters, and structural proteins [4]. It is very likely that there are changes in the body�s serum biomarkers of neuropathic pain before SCS and after SCS. Such a study would contribute greatly to the field of neuromodulation, as objective quantifiers of neuropathic pain control before and after SCS have not yet been investigated. Such definitive data regarding the effectiveness of SCS in relieving neuropathic pain and improving function will be important in future use of SCS.
In preparation for the launching of this study, the authors� goal with this transcript is to provide a review of the literature regarding known biomarkers for chronic neuropathic pain, and then prepare a role for biomarker analysis in the prediction of therapy success in SCS.
Data
The expression of certain genes is altered under chronic pain conditions. This alteration has helped provide an insight into the identification of potential biomarkers [5]. Current advanced research suggests that genetic expression of cytokines, positively or negatively, correlates with the experience of chronic pain. This negative or positive correlation primarily depends on the nature of the cytokine. Cytokines are signaling proteins which mediate the activation, differentiation, and proliferation of immune cells. They can be pro-inflammatory or anti-inflammatory. A misaligned balance between pro-inflammatory and anti-inflammatory cytokines has been common in most of the studies conducted (Table 1). Pro-inflammatory cytokines such as IL-1?, IL-6, IL-2, IL-33, CCL3, CXCL1, CCR5, and TNF-?, have been found to play significant roles in the amplification of chronic pain states. In studies involving discogenic pain, Complete Freund�s adjuvant (CFA)-induced discogenic pain in animal models has been observed to coincide with a sustained up-regulation of the above named cytokines [6]. In a more recent study, chronic constriction injury (CCI)-induced rats (neuropathic pain induction) were shown to have increased serum levels of CCL3 and CCR5. Even more interesting, an intrathecal injection of the anti-inflammatory cytokine, IL-4, and the CCL3-neutralizing antibody, reduced the CCI-induced neuropathic pain, estimated by a plantar test [7]. Other studies have also shown that the selective genetic impairment of the highlighted pro-inflammatory cytokines attenuated nerve-injury induced pain behavior, observed in neuropathic pain models [8]. Particularly, Zarpelon et al. revealed that CCI-induced rats showed reduced mechanical hyperalgesia when the IL-33 receptor gene, IL-33R (ST2), was knocked out, compared to the wild-type mice [9].
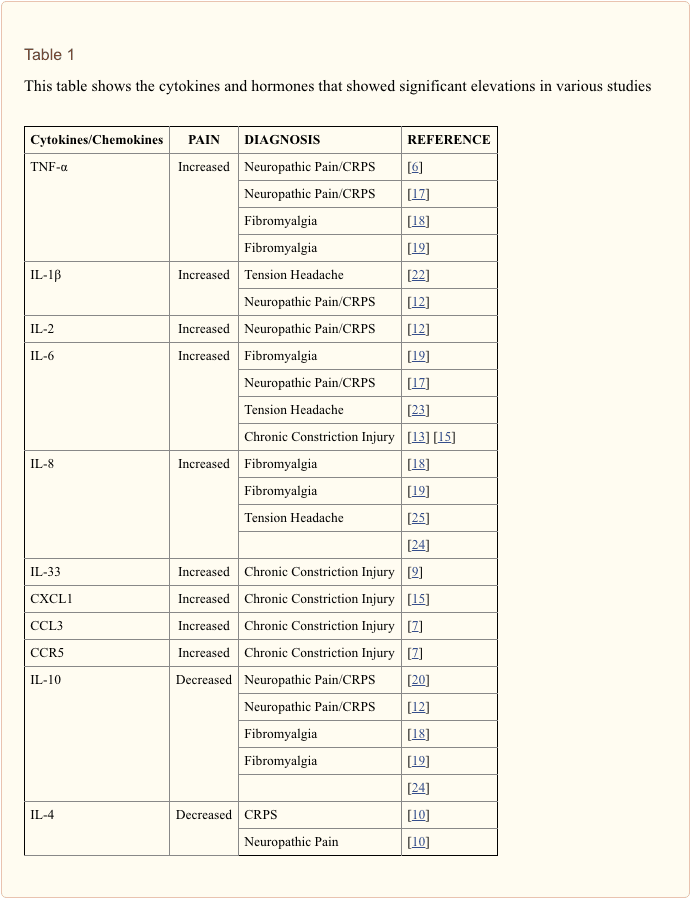
On the other hand, one study showed that blood levels of anti-inflammatory cytokines (such as IL-10 and IL-4) of Complex Regional Pain Syndrome (CRPS) patients were lower compared to the control [10]. A recent study also shows a distinction of the significant increases of pro-inflammatory cytokines based on the part of the back affected. There were more significant elevations (p<0.05, student�s t test) of pro-inflammatory cytokines in the plasma of lower back pain patients than in upper back patients, when compared to controls [11]. There has also been a study focusing on the levels of the aforementioned cytokines in patients with painful neuropathy in contrast with painless neuropathy and healthy control subjects. Patients with a painful neuropathy had about double the level of IL-2 expression (p = 0.001), TNF expression (p < 0.0001) and protein levels (p = 0.009) than the controls. The study further indicated there was about double the IL-2 and TNF level expression (p = 0.03; p = 0.001) and protein levels in painful neuropathy (p = 0.04; p = 0.04) than patients with painless neuropathy. On the contrary, levels of mRNA expression of the anti-inflammatory cytokines, IL-10 and IL-4 were considerably lower in patients with painful neuropathy than in patients with painless neuropathy (p =0.001) [12].
Several other studies, focused on the antagonist and agonist effects of some drugs targeting pro-inflammatory and anti-inflammatory cytokines, also have pointed out their significance with pain. Certain known analgesics were seen to reduce the levels of pro-inflammatory cytokines in the studies reviewed. There was a study about (CCI)-induced rats, in which case, this induced injury significantly, elevated the levels of pro-inflammatory cytokines, and decreased the serum levels of anti-inflammatory cytokines. Omeprazole, a known remedy for stomach pain, was observed to reduce the levels of pro-inflammatory cytokines (TNF-?, IL-1?, and IL-6) to normal, compared with the CCI control. It is important to note that this was while curbing the CCI-induced neuropathic pain, measured with the paw withdrawal latency [13]. Zhou et al. also highlighted the significance of certain drugs in determining the correlation between cytokines and neuropathic pain. CCI was again, used to induce neuropathic pain on rat models; and in turn, Paenoflorin, an established analgesic [14] was administered. Once Paenoflorin was introduced, significant decreases in serum levels of pro-inflammatory cytokines of CCI-induced rats (IL-1?, IL-6, TNF-?, and CXCL1) were observed, compared to the CCI-control [15]. The cytokines identified here, are the ones that showed correlation in various studies reviewed.
Even though cytokines are the key chronic pain biomarkers, according to the studies reviewed, there are still other proteins and nucleotides that have been observed to associate with chronic pain disorder. Two studies laid emphasis on regulatory microRNAs (miRNAs), which are small non-coding RNA molecules involved in post-transcriptional gene regulation. miRNAs achieve this by binding to mRNAs and degrading them or repressing their functions. Orlova et al. showed that 60% of CRPS patients in their study showed a significant down-regulation of 18 different miRNAs. The rest of the patients, however, showed variable (contradicting) miRNA levels. miRNA levels show variability, depending on the gene being regulated [5]. Tao et al. revealed that an increased inflammatory stimulation by the cytokine IL-1? in normal and osteoarthritis chondrocytes produced a significant down-regulation of the miRNA, miR-558, and a significant up-regulation of miR-21 in DRG neurons. A connection between IL-1? and miR-21 was attributed to AP-1, which is a transcription factor located in the promoter site of the mRNA, and is activated by IL-1? [4]. siRNAs have the same features as miRNAs, in the sense, that they are regulatory nucleotides. They also show variability, depending on the gene being regulated. SIRT1, a deacetylase, functions in regulating various pathways, including inflammation. It was observed that an intrathecal injection of SRT170, an SIRT1 agonist, reduced serum levels of NF-?B, a transcription factor for pro-inflammatory cytokines, in CCI-induced rat models. When SRT170-siRNA (a regulator of the regulator) was administered before SRT170, there was no agonistic effect [16].

Dr. Alex Jimenez’s Insight
A biomarker is most accurately defined as any measurement which demonstrates an interaction between a biological system and the possibility of a chemical, physical or biological hazard. However, biomarkers are often most commonly associated with medicine. In this setting, these can be utilized to determine the effects a particular treatment may have on a patient as well as for determining the risk a patient may have of developing certain health issues. An example of a diagnostic use of biomarkers includes the measurement of biomarkers in blood to evaluate the severity of a heart attack. In the same manner, blood samples can be analyzed and biomarkers can be measured in the instance of chronic pain.
Discussion
Chronic neuropathic pain affects a tremendous amount of the population. There are few effective therapies. However, outcomes are hard to determine due to the subjective nature of pain. We would like to devise a strategy that would establish objectivity of pain assessment. After review of various studies relating to pain biomarkers, we found that serum levels of pro-inflammatory cytokines and chemokines, such as IL-1?, IL-6, IL-2, IL-33, CCL3, CXCL1, CCR5, and TNF-?, were significantly up-regulated during chronic pain experience. On the other hand, anti-inflammatory cytokines such as IL-10 and IL-4 were found to show significant down-regulation during chronic pain state. Regulatory miRNAs, siRNAs, and deacetylases that coincide with these cytokines, also showed negative correlation, corresponding to the cytokine they were regulating.
The authors would like to apply this knowledge to SCS, a therapy for chronic neuropathic pain, in an attempt to develop a biomarker profile to help predict success. This study will be a prospective study including patients scheduled for SCS. One month before SCS surgery, patients will complete a survey assessing their subjective level of pain on the visual analog scale and subjective level of function. Patients will also have venipuncture performed, and serum will be analyzed for levels of pain biomarkers. After SCS surgery, patients will be followed at 6 more time points: 2 weeks, 1 month, 3 months, 6 months, 1 year, and 2 years. At each time point, the survey will be re-administered and blood work will be repeated. By evaluating patients pre-operatively and post-operatively, we will be able to assess subjective and objective levels of pain, allowing us to analyze trends in pain biomarkers in the context of patient-reported pain measurement. The duration of this study will be 4 years. Each subject will participate in this study for a total period of 25 months, which will allow us to follow these patients for 2 years postoperatively.
Conclusion
The review of various studies relating to inflammation- and/or nerve injury-induced chronic pain disorder led us to hypothesize that the application of the spinal cord stimulation procedure should relatively reduce serum pro-inflammatory cytokines, and relatively increase serum levels of anti-inflammatory cytokines. This should, in turn, help us understand the mechanism of spinal cord stimulation, thereby optimizing the efficaciousness of the procedure, and perhaps allow us to make predictions regarding therapy success. A follow-up prospective study regarding serum biomarker profile in SCS patients is currently being undertaken.
Footnotes
Author Disclosure: The authors declare no conflicts of interest.
Disclosure of Funding: This work was supported by grants from the University of Medicine and Dentistry of New Jersey and the National Institutes of Health, Bethesda, Maryland (grant numbers: NS072206, HL117684, and DA033390).
In conclusion,�diagnostic biomarkers have the potential of leading new personalized therapeutics in the treatment of chronic pain health issues, such as low back pain, disc degeneration and neuropathic pain. Several research studies like the ones above have been established to ultimately help healthcare professionals understand better ways to reduce or eliminate the pain and discomfort associated with these chronic pain problems. Furthermore, biomarkers can be essential diagnostic tools for the evaluation and treatment of a variety of health issues. Information referenced from the National Center for Biotechnology Information (NCBI).�The scope of our information is limited to chiropractic as well as to spinal injuries and conditions. To discuss the subject matter, please feel free to ask Dr. Jimenez or contact us at�915-850-0900�.
Curated by Dr. Alex Jimenez
Additional Topics: Back Pain
Back pain is one of the most prevalent causes for disability and missed days at work worldwide. As a matter of fact, back pain has been attributed as the second most common reason for doctor office visits, outnumbered only by upper-respiratory infections. Approximately 80 percent of the population will experience some type of back pain at least once throughout their life. The spine is a complex structure made up of bones, joints, ligaments and muscles, among other soft tissues. Because of this, injuries and/or aggravated conditions, such as herniated discs, can eventually lead to symptoms of back pain. Sports injuries or automobile accident injuries are often the most frequent cause of back pain, however, sometimes the simplest of movements can have painful results. Fortunately, alternative treatment options, such as chiropractic care, can help ease back pain through the use of spinal adjustments and manual manipulations, ultimately improving pain relief.

EXTRA IMPORTANT TOPIC: Low Back Pain Management
MORE TOPICS: EXTRA EXTRA:�Chronic Pain & Treatments
Blank
References
Close Accordion
General Disclaimer, Licenses and Board Certifications *
Professional Scope of Practice *
The information herein on "Lab Biomarkers for Chronic Pain" is not intended to replace a one-on-one relationship with a qualified health care professional or licensed physician and is not medical advice. We encourage you to make healthcare decisions based on your research and partnership with a qualified healthcare professional.
Blog Information & Scope Discussions
Welcome to El Paso's Premier Wellness and Injury Care Clinic & Wellness Blog, where Dr. Alex Jimenez, DC, FNP-C, a Multi-State board-certified Family Practice Nurse Practitioner (FNP-BC) and Chiropractor (DC), presents insights on how our multidisciplinary team is dedicated to holistic healing and personalized care. Our practice aligns with evidence-based treatment protocols inspired by integrative medicine principles, similar to those on this site and on our family practice-based chiromed.com site, focusing on naturally restoring health for patients of all ages.
Our areas of multidisciplinary practice include Wellness & Nutrition, Chronic Pain, Personal Injury, Auto Accident Care, Work Injuries, Back Injury, Low Back Pain, Neck Pain, Migraine Headaches, Sports Injuries, Severe Sciatica, Scoliosis, Complex Herniated Discs, Fibromyalgia, Complex Injuries, Stress Management, Functional Medicine Treatments, and in-scope care protocols.
Our information scope is multidisciplinary, focusing on musculoskeletal and physical medicine, wellness, contributing etiological viscerosomatic disturbances within clinical presentations, associated somato-visceral reflex clinical dynamics, subluxation complexes, sensitive health issues, and functional medicine articles, topics, and discussions.
We provide and present clinical collaboration with specialists from various disciplines. Each specialist is governed by their professional scope of practice and their jurisdiction of licensure. We use functional health & wellness protocols to treat and support care for musculoskeletal injuries or disorders.
Our videos, posts, topics, and insights address clinical matters and issues that are directly or indirectly related to our clinical scope of practice.
Our office has made a reasonable effort to provide supportive citations and has identified relevant research studies that support our posts. We provide copies of supporting research studies upon request to regulatory boards and the public.
We understand that we cover matters that require an additional explanation of how they may assist in a particular care plan or treatment protocol; therefore, to discuss the subject matter above further, please feel free to ask Dr. Alex Jimenez, DC, APRN, FNP-BC, or contact us at 915-850-0900.
We are here to help you and your family.
Blessings
Dr. Alex Jimenez, DC, MSACP, APRN, FNP-BC*, CCST, IFMCP, CFMP, ATN
email: [email protected]
Multidisciplinary Licensing & Board Certifications:
Licensed as a Doctor of Chiropractic (DC) in Texas & New Mexico*
Texas DC License #: TX5807, Verified: TX5807
New Mexico DC License #: NM-DC2182, Verified: NM-DC2182
Multi-State Advanced Practice Registered Nurse (APRN*) in Texas & Multi-States
Multi-state Compact APRN License by Endorsement (42 States)
Texas APRN License #: 1191402, Verified: 1191402 *
Florida APRN License #: 11043890, Verified: APRN11043890 *
Colorado License #: C-APN.0105610-C-NP, Verified: C-APN.0105610-C-NP
New York License #: N25929, Verified N25929
License Verification Link: Nursys License Verifier
* Prescriptive Authority Authorized
ANCC FNP-BC: Board Certified Nurse Practitioner*
Compact Status: Multi-State License: Authorized to Practice in 40 States*
Graduate with Honors: ICHS: MSN-FNP (Family Nurse Practitioner Program)
Degree Granted. Master's in Family Practice MSN Diploma (Cum Laude)
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card
Licenses and Board Certifications:
DC: Doctor of Chiropractic
APRNP: Advanced Practice Registered Nurse
FNP-BC: Family Practice Specialization (Multi-State Board Certified)
RN: Registered Nurse (Multi-State Compact License)
CFMP: Certified Functional Medicine Provider
MSN-FNP: Master of Science in Family Practice Medicine
MSACP: Master of Science in Advanced Clinical Practice
IFMCP: Institute of Functional Medicine
CCST: Certified Chiropractic Spinal Trauma
ATN: Advanced Translational Neutrogenomics
Memberships & Associations:
TCA: Texas Chiropractic Association: Member ID: 104311
AANP: American Association of Nurse Practitioners: Member ID: 2198960
ANA: American Nurse Association: Member ID: 06458222 (District TX01)
TNA: Texas Nurse Association: Member ID: 06458222
NPI: 1205907805
| Primary Taxonomy | Selected Taxonomy | State | License Number |
|---|---|---|---|
| No | 111N00000X - Chiropractor | NM | DC2182 |
| Yes | 111N00000X - Chiropractor | TX | DC5807 |
| Yes | 363LF0000X - Nurse Practitioner - Family | TX | 1191402 |
| Yes | 363LF0000X - Nurse Practitioner - Family | FL | 11043890 |
| Yes | 363LF0000X - Nurse Practitioner - Family | CO | C-APN.0105610-C-NP |
| Yes | 363LF0000X - Nurse Practitioner - Family | NY | N25929 |
Dr. Alex Jimenez, DC, APRN, FNP-BC*, CFMP, IFMCP, ATN, CCST
My Digital Business Card









