
by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Arthritis, Arthropathies, Diets, Functional Medicine, Health, Holistic Medicine, Natural Health, Nutrition, Supplements, Vitamins, Weight Loss, Wellness
Our diet can significantly affect inflammation in our bodies. Several foods can increase inflammation while other foods can reduce inflammation. According to healthcare professionals, a diet that is high in sugar may be associated with chronic inflammation. A systematic review in 2018 demonstrated that eating excess sugar can ultimately cause inflammation and a variety of other health issues, such as diabetes. Another 2014 research study showed that people who decreased their consumption of sugary or sweetened drinks had reduced inflammation. These research findings support the theory that eating excess sugar can cause chronic inflammation and various other diseases, including diabetes.
How Sugar Can Cause Inflammation
Healthcare professionals have tried to understand how eating excess sugar can cause chronic inflammation. Sugar triggers the production of free fatty acids in the liver. When the human body digests these free fatty acids, the resulting compounds can trigger inflammation. Different types of sugar may also cause more inflammation. By way of instance, one research study found that fructose can cause more inflammation than glucose. However, a systematic review found that fructose didn’t cause more inflammation than glucose. Therefore, further research studies are still required to determine which types of sugar may cause more inflammation. Symptoms associated with chronic inflammation can include:
- pain and fatigue
- sleeping problems or insomnia
- anxiety, depression, and other mood disorders
- digestive problems like acid reflux, constipation, and/or diarrhea
- weight gain or obesity
- constant infections
People with chronic inflammation may also have an increased risk of developing a variety of other health issues, including diabetes and dementia. Chronic inflammation in older adults may also be associated with an increased risk of death.
Health Issues Caused by Chronic Inflammation
Observational research studies in humans have associated diets with high added sugar and refined carbohydrates to the increased risk of developing a variety of health issues, including diabetes, IBD, liver disease, dementia, and arthritis.
Diabetes
Research studies showed a connection between the increased consumption of added sugar and type 2 diabetes. A large analysis that included over 38,000 participants found that simply consuming one serving of sweetened drinks or beverages on a regular basis was associated with an 18 percent increased risk of developing type 2 diabetes. Another research study found that increasing the consumption of high-fructose corn syrup was also associated with diabetes.
Other Diseases
Increased consumption of added sugar and refined carbohydrates has also been associated with the development of other diseases, such as arthritis, inflammatory bowel disease, liver disease, and dementia. Furthermore, excess fructose consumption has been associated with non-alcoholic fatty liver disease. Healthcare professionals believe this may be due to a combination of ongoing low-grade inflammation, increased gut permeability, and bacterial overgrowth in the gut.
Other Foods That Can Cause Inflammation
- sugary foods like pastries, desserts, and chocolate
- saturated fats from processed meats and dairy products
- trans fats found in fast, fried, foods
- vegetable and seed oils
- refined carbohydrates
- excessive alcohol
- MSG in prepared Asian foods and deli meats
For information regarding how excess sugar can cause chronic inflammation and various other health issues like diabetes, please review this article:
Diet can affect inflammation in our bodies. Several foods can increase inflammation while other foods can reduce inflammation. A diet that is high in sugar may be associated with inflammation. Numerous research studies have demonstrated that eating excess sugar can ultimately cause chronic inflammation and various other diseases, including diabetes. Because sugar triggers the production of free fatty acids in the liver, it can also trigger inflammation. Excess sugar can cause chronic inflammation. Different types of sugar may also cause different amounts of inflammation. There are many symptoms associated with chronic inflammation, including pain, fatigue, obesity, anxiety, and depression, among others. Inflammation can lead to a variety of health issues, such as diabetes and arthritis. Although excess sugar is associated with chronic inflammation, other foods like saturated fats and refined carbohydrates can also cause health issues. In the following article, we discuss how sugar can cause inflammation and a variety of other health issues, such as diabetes, in the human body. – Dr. Alex Jimenez D.C., C.C.S.T. Insights

Sea Green Smoothie
Servings: 1
Cook time: 5-10 minutes
� 1/2 cup cantaloupe, cubed
� 1/2 banana
� 1 handful of kale or spinach
� 1 handful of Swiss chard
� 1/4 avocado
� 2 teaspoons spirulina powder
� 1 cup of water
� 3 or more ice cubes
Blend all ingredients in a high-speed blender until completely smooth and enjoy!

Leafy Greens Hold the Key to Gut Health
A unique type of sugar found in leafy greens can help feed our beneficial gut bacteria. Sulfoquinovose (SQ) is the only known sugar molecule to be made up of sulfur, an extremely essential mineral in the human body. The human body uses sulfur to produce enzymes, proteins, and a variety of hormones as well as antibodies for our cells. A fast and easy way to get leafy greens into your diet is to toss a couple of handfuls of them into a delicious smoothie!
The scope of our information is limited to chiropractic, musculoskeletal, physical medicines, wellness, and sensitive health issues and/or functional medicine articles, topics, and discussions. We use functional health & wellness protocols to treat and support care for injuries or disorders of the musculoskeletal system. Our posts, topics, subjects, and insights cover clinical matters, issues, and topics that relate and support directly or indirectly our clinical scope of practice.* Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. We understand that we cover matters that require an additional explanation as to how it may assist in a particular care plan or treatment protocol; therefore, to further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900. The provider(s) Licensed in Texas*& New Mexico*�
Curated by Dr. Alex Jimenez D.C., C.C.S.T.
References:
- Spritzler, Franziska. �6 Foods That Cause Inflammation.� Healthline, Healthline Media, 12 Nov. 2019, www.healthline.com/nutrition/6-foods-that-cause-inflammation#1.
- Caporuscio, Jessica. �Does Sugar Cause Inflammation? What the Research Says.� Medical News Today, MediLexicon International, 19 Sept. 2019, www.medicalnewstoday.com/articles/326386.
- Brown, Mary Jane. �Does Sugar Cause Inflammation in the Body?� Healthline, Healthline Media, 12 Nov. 2017, www.healthline.com/nutrition/sugar-and-inflammation.
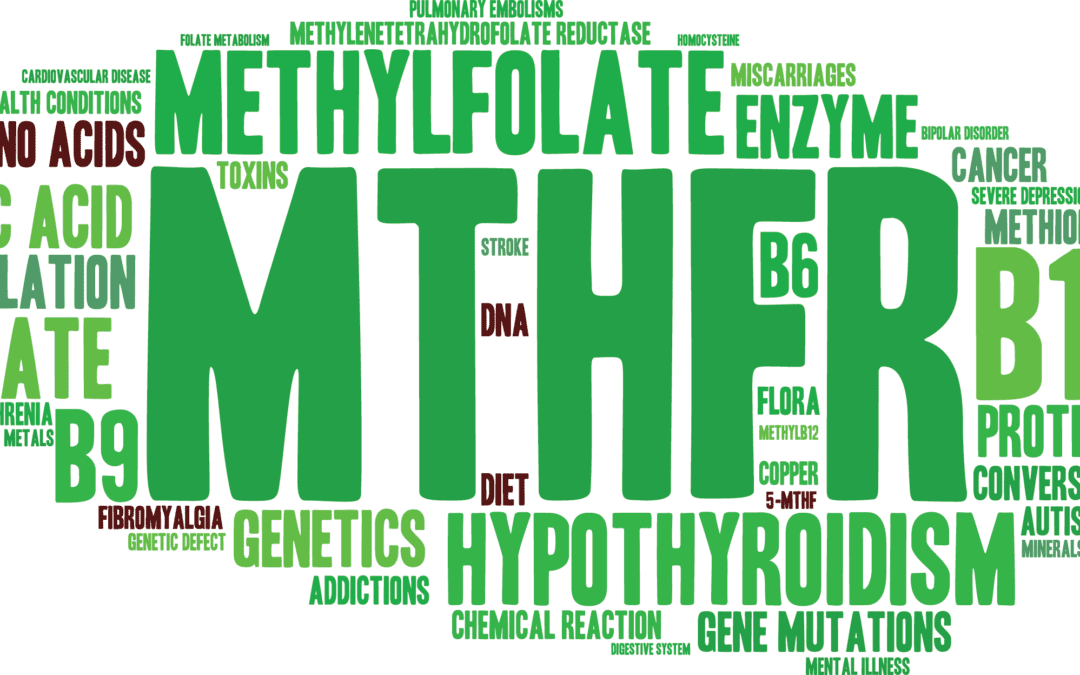
by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Diets, Epigenetic's, Functional Medicine, Health, Natural Health, Nutrition, Nutritional Genomics, Supplements, Vitamins, Wellness
The MTHFR or methylenetetrahydrofolate reductase gene is well-known due to a genetic mutation that may cause high homocysteine levels and low folate levels in the bloodstream, among other essential nutrients. Healthcare professionals believe that a variety of health issues, such as inflammation, may be associated with an MTHFR gene mutation. In the following article, we will discuss the MTHFR gene mutation and how it can ultimately affect your overall health.
What is an MTHFR Gene Mutation?
People can have single or multiple mutations, as well as neither, on the MTHFR gene. The different mutations are often referred to as “variants”. A variant occurs when the DNA of a specific part of a gene is different or varies from person to person. People that have a heterozygous or single variant of the MTHFR gene mutation have a decreased risk of developing health issues like inflammation and chronic pain, among other diseases. Moreover, healthcare professionals also believe that people that have homozygous or multiple variants of the MTHFR gene mutation may ultimately have an increased risk of disease. There are two MTHFR gene mutation variants. These specific variants include:
- C677T. Approximately 30 to 40 percent of people in the United States have a mutation at gene position C677T. About 25 percent of Hispanics and about 10 to 15 percent of Caucasians are homozygous for this variant.
- A1298C. There are limited research studies for this variant. A 2004 study focused on 120 blood donors of Irish heritage. Of the donors, 56 or 46.7 percent were heterozygous for this variant and 11 or 14.2 percent were homozygous.
- Both C677T and A1298C. It�s also possible for people to have both C677T and A1298C MTHFR gene mutation variations, which includes one copy of each.
What are the Symptoms of an MTHFR Gene Mutation?
Symptoms of an MTHFR gene mutation can be different from person to person and from variant to variant. It’s important to remember that further research around MTHFR gene mutation variants and their effects on health are still needed. Evidence regarding how MTHFR gene mutation variants are associated with a variety of other health issues is currently lacking or it has been disproven. Conditions that have been suggested to be associated with MTHFR variants include:
- anxiety
- depression
- bipolar disorder
- schizophrenia
- migraines
- chronic pain and fatigue
- nerve pain
- recurrent miscarriages in women of child-bearing age
- pregnancies with neural tube defects, like spina bifida and anencephaly
- cardiovascular and thromboembolic diseases (blood clots, stroke, embolism, and heart attacks)
- acute leukemia
- colon cancer
What is the MTHFR Diet?
According to healthcare professionals, eating foods with high amounts of folate may help naturally support low folate levels in the bloodstream associated with MTHFR gene mutation variants.�Good food choices can include:
- fruits, such as strawberries, raspberries, grapefruit, cantaloupe, honeydew, banana.
- juices like orange, canned pineapple, grapefruit, tomato, or other vegetable juice
- veggies, such as spinach, asparagus, lettuce, beets, broccoli, corn, Brussels sprouts, and bok choy
- proteins, including cooked beans, peas, and lentils
- peanut butter
- sunflower seeds
People with MTHFR gene mutations may also want to avoid eating foods that have the synthetic form of folate, folic acid, however, the evidence is not clear if that�s beneficial or necessary. Supplementation may still be recommended for people with MTHFR gene mutation variants. Furthermore, always make sure to check the labels of the foods you buy, as this vitamin is added to many enriched grains like pasta, cereals, bread, and commercially produced flours.
For information regarding the MTHFR and its effects on health issues like cancer, please review this article:
Folate, Methyl-Related Nutrients, Alcohol, and the MTHFR 677C >T Polymorphism Affect Cancer Risk: Intake Recommendations
MTHFR, or methylenetetrahydrofolate reductase, gene mutations may cause high homocysteine levels and low folate levels in the bloodstream. We believe that a variety of health issues, such as inflammation, may be associated with an MTHFR gene mutation. People can have single or multiple MTHFR gene mutations, as well as neither. The different mutations are often referred to as “variants”. People that have a heterozygous or single variant of the MTHFR gene mutation have a decreased risk of developing health issues like inflammation and chronic pain. Moreover, doctors also believe that people that have homozygous or multiple variants of the MTHFR gene mutation may ultimately have an increased risk of disease. The two MTHFR gene mutation variants are�C677T, A1298C, or both C677T and A1298C. Symptoms of an MTHFR gene mutation can be different from person to person and from variant to variant. Following what is referred to as the MTHFR diet can ultimately help improve overall health in people with MTHFR gene mutation variants. Also, adding these foods into a smoothie can be an easy way to add them into your diet. – Dr. Alex Jimenez D.C., C.C.S.T. Insights

Protein Power Smoothie
Serving: 1
Cook time: 5 minutes
� 1 scoop protein powder
� 1 tablespoon ground flaxseed
� 1/2 banana
� 1 kiwi, peeled
� 1/2 teaspoon cinnamon
� Pinch of cardamom
� Non-dairy milk or water, enough to achieve desired consistency
Blend all ingredients in a high-powered blender until completely smooth. Best served immediately!

Leafy Greens Hold the Key to Gut Health
A unique type of sugar found in leafy greens can help feed our beneficial gut bacteria. Sulfoquinovose (SQ) is the only known sugar molecule to be made up of sulfur, an extremely essential mineral in the human body. The human body uses sulfur to produce enzymes, proteins, and a variety of hormones as well as antibodies for our cells. A fast and easy way to get leafy greens into your diet is to toss a couple of handfuls of them into a delicious smoothie!
The scope of our information is limited to chiropractic, musculoskeletal, physical medicines, wellness, and sensitive health issues and/or functional medicine articles, topics, and discussions. We use functional health & wellness protocols to treat and support care for injuries or disorders of the musculoskeletal system. Our posts, topics, subjects and insights cover clinical matters, issues, and topics that relate and support directly or indirectly our clinical scope of practice.* Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. We understand that we cover matters that require additional explanation as how it may assist in a particular care plan or treatment protocol; therefore, to further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at�915-850-0900. The provider(s) Licensed in Texas*& New Mexico*�
Curated by Dr. Alex Jimenez D.C., C.C.S.T.
References:
- Marcin, Ashley. �What You Need to Know About the MTHFR Gene.� Healthline, Healthline Media, 6 Sept. 2019, www.healthline.com/health/mthfr-gene#variants.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Diets, Functional Medicine, Health, Holistic Medicine, Natural Health, Nutrition, Supplements, Weight Loss, Wellness
Calories are defined as a measurement of the energy our body produces from the foods we eat. However, not all calories are created equal. If we were to eat nothing but spoonfuls of sugar all-day, by way of instance, our health would tremendously deteriorate because there simply aren’t enough nutrients in those calories from sugar. The human body needs a variety of nutrients, vitamins, minerals, and many other compounds in order to function properly.
The foods we eat are made up of calories as well as complex mixtures of nutrients, fiber, and additives. This can ultimately affect the hormones that regulate our hunger, known as leptin, and those that manage how we burn or store calories to be used for energy, known as insulin. Our bodies are naturally programmed to protect us against long-term starvation by storing excess calories as fat. Eating “bad” calories in excess amounts can ultimately lead to obesity.
In a research study, a group of people was given the same amount of calories but from different food sources. The participants had no significant weight gain, regardless of whether the calories were from carbohydrates, proteins, fats, or any other combination of nutrients. However, environmental factors, such as an individual’s hormonal balance, emotions, and cravings were not taken into consideration. It’s important to understand how calories can affect your health.
Good Calories vs Bad Calories
Excess calories from processed foods are stored as fat which can lead to obesity. In the United States, obesity is the main cause of health issues like insulin resistance. Insulin is a hormone that regulates blood sugar levels. It is naturally produced in the pancreas and helps move excess glucose from the bloodstream into the cells to be used for energy. When the pancreas recognizes high blood sugar levels, it creates more insulin to reduce glucose.
However, this can diminish the pancreas of insulin-producing cells which can eventually cause insulin resistance or impaired insulin sensitivity. If the pancreas can’t produce enough insulin, it can lead to prediabetes or type 2 diabetes. Excess calories from sugar and processed foods can also cause inflammation which may also lead to chronic pain. So what can we do to prevent these health issues? The answer is simple: eat complex carbohydrates, lean protein, and healthy fats.
Replace highly processed carbohydrates that can increase blood sugar levels and insulin, with vegetables, beans, and whole grains. When it comes to eating complex carbohydrates like whole grains, the less processed the better! Consider eating stone-ground whole wheat, quinoa, oats, and brown rice. Then, choose lean proteins, such as fish and chicken. as well as healthy fats that come from plant sources, such as nuts, olive oil, and avocado, among others.
Below, we will compare the calories in common foods and drinks to demonstrate the differences and similarities in good calories vs bad calories:�


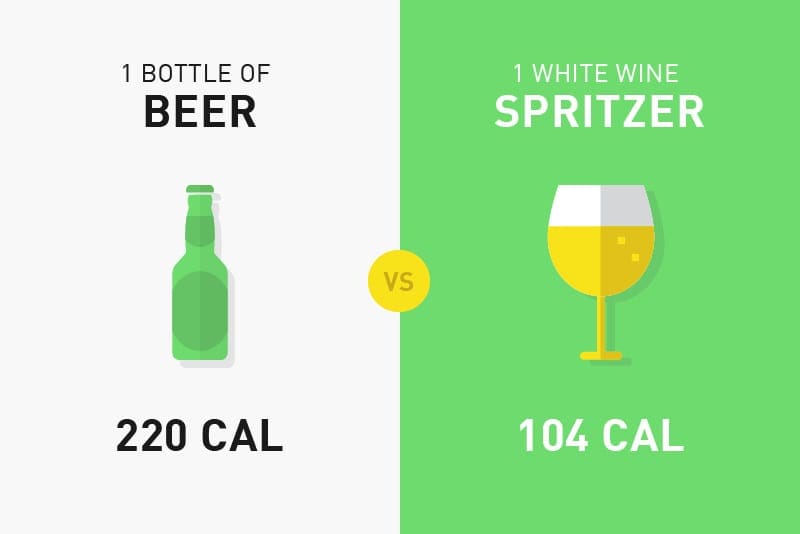


Can you tell which are the good calories and which are the bad calories? It�s important to follow the principle of �clean eating� and choose unprocessed foods in the purest forms instead of processed foods. This includes foods like fruits, vegetables, legumes, nuts, or eggs. You can eat these foods without worrying too much about your daily caloric intake limit. Eating a variety of these is essential in order to provide your body with the nutrients it needs to function properly.
Bad calories include processed foods which follow exactly the opposite principle of “clean eating”. Foods with high amounts of sugar and fast food offers you almost no nutrients but a lot of what we call “empty calories”. If you�re trying to lose weight to manage insulin resistance associated with type 2 diabetes, you�ll have to pay attention to your �bad� calorie intake.
For more information regarding the effects of good calories vs bad calories on obesity, please review this article:
Is the calorie concept a real solution to the obesity epidemic?
Our body needs nutrients, vitamins, minerals, and many other compounds from calories in order to function properly. Calories are a measurement of the energy our body produces from the foods we eat. But, not all calories are created equal. Eating bad calories vs good calories can affect the hormones that regulate our hunger and those that manage how we burn or store calories to be used for energy. Moreover, eating “bad” calories in excess amounts can cause obesity. It’s important to understand how calories can affect your health. In the United States, obesity is the main cause of health issues like insulin resistance and type 2 diabetes. Excess bad calories can also cause inflammation which may cause chronic pain. Eating complex carbohydrates, lean protein, and healthy fats can help people lose weight and prevent as well as control health issues like insulin resistance and type 2 diabetes. Learning to identify good calories and bad calories is a helpful strategy for people who want to improve their overall health. Adding healthy foods to a smoothie can also be a fast and easy way to include good calories into your diet. – Dr. Alex Jimenez D.C., C.C.S.T. Insights

Zesty Beet Juice
Servings: 1
Cook time: 5-10 minutes
� 1 grapefruit, peeled and sliced
� 1 apple, washed and sliced
� 1 whole beet, and leaves if you have them, washed and sliced
� 1-inch knob of ginger, rinsed, peeled and chopped
Juice all ingredients in a high-quality juicer. Best served immediately.

Add Nasturtium to Your Smoothies
Adding nasturtium flowers and leaves to any smoothie can add extra nutrients. These lovely plants are easy to grow and the entire plant is edible. Nasturtium leaves are high in vitamin C, which is essential for a healthy immune system, and they also contain calcium, potassium, phosphorus, zinc, copper, and iron. According to healthcare professionals, the extract from the flowers and leaves have antimicrobial, antifungal, hypotensive, expectorant, and anticancer effects. Antioxidants in garden nasturtium occur due to its high content of compounds such as anthocyanins, polyphenols, and vitamin C. Due to its rich phytochemical content and unique elemental composition, the garden nasturtium may be used in the treatment of a variety of health issues, including respiratory and digestive problems. Not to mention, the flowers and leaves look absolutely lovely in smoothies.
The scope of our information is limited to chiropractic, musculoskeletal, physical medicines, wellness, and sensitive health issues and/or functional medicine articles, topics, and discussions. We use functional health & wellness protocols to treat and support care for injuries or disorders of the musculoskeletal system. Our posts, topics, subjects and insights cover clinical matters, issues, and topics that relate and support directly or indirectly our clinical scope of practice.* Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. We understand that we cover matters that require additional explanation as how it may assist in a particular care plan or treatment protocol; therefore, to further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at�915-850-0900. The provider(s) Licensed in Texas*& New Mexico*�
Curated by Dr. Alex Jimenez D.C., C.C.S.T.
References:
- Glassman, Keri. �The Difference Between Good and Bad Calories.� Women’s Health, Women’s Health Media, 11 June 2019, www.womenshealthmag.com/food/a19930112/the-difference-between-good-and-bad-calories/.
- Denner, Julia. �Good Calories Vs. Bad Calories >> The Difference Matters.� Adidas Runtastic Blog, Adidas Runtastic Blog Media, 9 Sept. 2019, www.runtastic.com/blog/en/good-calories-vs-bad-calories/.
- Taubes, Gary. �Good Calories Bad Calories: Fats, Carbs, and the Controversial Science of Diet and Health.� CrossFit, CrossFit Media, 31 Jan. 2020, www.crossfit.com/health/good-calories-bad-calories.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Diets, Epigenetic's, Functional Medicine, Health, Holistic Medicine, Natural Health, Nutrition, Nutritional Genomics, Supplements, Wellness
Nutrition is considered to be one of the most well-understood environmental factors associated with changes in the epigenome. Nutrients in the foods we eat are processed by our metabolism and turned into energy. One metabolic pathway, however, is responsible for producing methyl groups or fundamental epigenetic marks that regulate our gene expression. Essential nutrients, such as B vitamins, SAM-e (S-Adenosyl methionine), and folic acid are important components in this methylation process. Diets with high amounts of these essential nutrients can quickly change gene expression, especially during early development. In the following article, we will discuss the connection between nutrition and the epigenome.
Nutrigenomics and Health
Healthcare professionals discuss that when it comes to dealing with health issues like inflammation and chronic pain, understanding how nutrigenomics affects our overall health is important. Nutritional genomics, or nutrigenomics, is a science that studies the relationship between nutrition, health, and the genome. Researchers in the nutrigenomics field believe that changes in epigenetic marks may be associated with a variety of health issues, including inflammation or the development of diseases like obesity, heart problems, and cancer. Studies have demonstrated that we may be able to control the effects of the nutrients we eat in order to change gene expression associated with various health issues.
Approximately more than 1 out of 3 adults in the United States have been diagnosed with obesity which ultimately increases the risk of a variety of health issues, including prediabetes and type 2 diabetes, among other diseases. Previous studies have demonstrated that changes in epigenetic marks during early development may even predispose individuals to obesity. Moreover, changes in epigenetic marks were also demonstrated to affect metabolic pathways that may increase the risk of prediabetes and type 2 diabetes. Healthcare professionals in the nutrigenomics field have created new ways to be able to better find balance through a wholesome understanding of nutrition and the epigenome.
“An epigenetic test can provide data that is useful for healthcare professionals. It may also offer information about how certain metabolic pathways are affected by essential nutrients, such as vitamins and minerals”.
What is the Epigenetics Diet?
The term “epigenetics diet” was first coined by Dr. Trygve Tollefsbol in 2011. It is medically defined as a group of compounds, such as resveratrol in red grapes, genistein in soybeans, isothiocyanates in broccoli, and many other well-known types of foods, which have been demonstrated to help change epigenomic marks and gene expression. According to researchers, the epigenetics diet can prevent the progression of tumors by regulating enzymes that control these epigenomic marks and gene expression, including DNA methyltransferases, histone deacetylases, and certain non-coding RNAs. Several types of foods included in the epigenetics diet are demonstrated in the following infographic:

Researchers used recently advanced technologies that demonstrated how several bioactive compounds may aggravate damage to the epigenome caused by environmental pollutions. By way of instance, dietary supplementation with methyl donors, such as vitamin B12, choline, and folate, among others, as well as the isoflavone genistein, can regulate changes to epigenome marks and gene expression caused by bisphenol A, a hormone-disrupting chemical. B vitamins may also prevent the loss of DNA methylation caused by air pollution. According to these same studies, dietary supplementation with folic acid has also been demonstrated to help prevent the negative side-effects caused by heavy metals.
We believe that foods in the epigenetics diet could be used to counteract changes to gene expression and epigenomic marks caused by environmental pollution. Environmental pollutants in several types of foods, such as pesticides in fruits like strawberries and leafy greens like spinach, bisphenol A in the plastic containers of foods and drinks, dioxins in fatty foods, polycyclic aromatic hydrocarbons produced when meat is grilled or smoked at high temperatures, and mercury in several types of seafood like king mackerel and swordfish, have been associated with changes to epigenomic marks and gene expression. Those exposures, especially during early development, may cause various health issues.
For more information regarding the connection between nutrition and the epigenome, please review this article:
Nutrition and the Epigenome
Nutrition is one of the most understood environmental factors associated with changes in epigenomic marks and gene expression. Essential nutrients found in the different types of foods we eat are metabolized and turned into molecules in order to be used for energy by the human body. One metabolic pathway is responsible for creating methyl groups, important epigenetic marks that regulate our gene expression and epigenomic marks. Essential nutrients, including B vitamins, SAM-e (S-Adenosyl methionine), and folic acid are fundamental components in DNA methylation. Diets that are rich in these essential nutrients can quickly change epigenetic marks and gene expression, especially during early development. Furthermore, adding a variety of good foods to a smoothie can be a fast and easy way to add essential nutrients to your diet. Below is a fast and easy smoothie recipe to help feed your genes. – Dr. Alex Jimenez D.C., C.C.S.T. Insights

Ginger Greens Juice
Servings: 1
Cook time: 5-10 minutes
� 1 cup pineapple cubes
� 1 apples, sliced
� 1-inch knob of ginger, rinsed, peeled and chopped
� 3 cups kale, rinsed and roughly chopped or ripped
� 5 cups Swiss chard, rinsed and roughly chopped or ripped
Juice all ingredients in a high-quality juicer. Best served immediately.

Add Nasturtium to Your Smoothies
Adding nasturtium flowers and leaves to any smoothie can add extra nutrients. These lovely plants are easy to grow and the entire plant is edible. Nasturtium leaves are high in vitamin C, which is essential for a healthy immune system, and they also contain calcium, potassium, phosphorus, zinc, copper, and iron. According to healthcare professionals, the extract from the flowers and leaves have antimicrobial, antifungal, hypotensive, expectorant, and anticancer effects. Antioxidants in garden nasturtium occur due to its high content of compounds such as anthocyanins, polyphenols, and vitamin C. Due to its rich phytochemical content and unique elemental composition, the garden nasturtium may be used in the treatment of a variety of health issues, including respiratory and digestive problems. Not to mention, the flowers and leaves look absolutely lovely in smoothies.
The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues or functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or disorders of the musculoskeletal system. Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. To further discuss the subject matter above, please feel free to ask�Dr. Alex Jimenez�or contact us at�915-850-0900.
Curated by Dr. Alex Jimenez D.C., C.C.S.T.
References:
- Kirkpatrick, Bailey. �Epigenetics, Nutrition, and Our Health: How What We Eat Could Affect Tags on Our DNA.� What Is Epigenetics?, What Is Epigenetics? Media, 11 May 2018, www.whatisepigenetics.com/epigenetics-nutrition-health-eat-affect-tags-dna/.
- Li, Shizhao, et al. �The Epigenetics Diet: A Barrier against Environmental Pollution.� On Biology, BMC Media, 23 May 2019, blogs.biomedcentral.com/on-biology/2019/05/20/the-epigenetics-diet-a-barrier-against-environmental-pollution/.
- Learn. Genetics Staff. �Nutrition & the Epigenome.� Learn. Genetics, Learn. Genetics Media, learn.genetics.utah.edu/content/epigenetics/nutrition/.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Diets, Functional Medicine, Health, Holistic Medicine, Natural Health, Nutrition, Supplements, Vitamins, Weight Loss, Wellness
Insulin is an essential hormone that regulates blood sugar levels. It is naturally produced in the pancreas and helps move excess glucose from the bloodstream into the cells to be used for energy. When the pancreas recognizes high blood sugar levels, it creates more insulin to reduce glucose. However, this can diminish the pancreas of insulin-producing cells which can eventually cause insulin resistance or impaired insulin sensitivity. If the pancreas can’t produce enough insulin, it can lead to prediabetes or type 2 diabetes. In the following article, we will discuss natural ways to improve insulin resistance or impaired insulin sensitivity to prevent and regulate prediabetes and type 2 diabetes, among other health issues.
Foods to Avoid with Insulin Resistance
If you have insulin resistance or impaired insulin sensitivity associated with prediabetes, type 2 diabetes, or any other health issue, there are several types of foods that can increase blood sugar levels. Frequently eating foods with high glucose content can diminish the insulin-producing cells that can ultimately affect the human body’s ability to produce enough insulin. When this occurs, high blood sugar levels remain elevated which can ultimately cause prediabetes and type 2 diabetes as well as lead to a variety of other health issues, including damage to organs such as the eyes and kidneys or limbs (neuropathy). Avoid eating the following types of foods if you have insulin resistance or impaired insulin sensitivity:
- fried foods
- processed snacks and foods
- dairy products from cows, such as milk
- foods high in saturated fats, such as butter, and salt pork
- refined grains, such as white rice, pasta, bread, and flour-based foods
- sugary sweets and pastries, such as ice cream, chocolate bars, and cupcakes
- starchy vegetables, such as corn, potatoes and yams (without skin), and pumpkin
- sweetened drinks or beverages, such as fruit juices, fountain drinks, and sodas
- alcohol, such as beer and grain alcohol, in large quantities
Foods to Eat with Insulin Resistance
Many people are commonly deficient in essential nutrients, such as calcium, potassium, magnesium, and fiber. These nutrients are necessary for regulating blood sugar levels. People with insulin resistance or impaired insulin sensitivity, or any other health issue, including prediabetes or type 2 diabetes, should eat foods that have plenty of these essential nutrients. According to the American Diabetes Association, people with insulin resistance or impaired insulin sensitivity can eat from any of the basic food groups, however, it’s fundamental for individuals to understand which types of foods can increase blood glucose levels. Eat from the following types of foods if you have insulin resistance or impaired insulin sensitivity:
- antioxidant-rich foods, such as berries
- citrus fruits, such as oranges, lemons, and limes
- non-starchy vegetables, such as dark leafy greens, peppers, and broccoli
- protein-rich foods, such as legumes, nuts, soy, fish, and lean meats
- high-fiber foods, including beans, and lentils
- omega-3 fatty acid-rich foods, such as sardines, herring, and salmon
- certain types of whole grains, such as oats, quinoa, and barley
- water, especially as a substitute for sweetened drinks and
- unsweetened teas
Exercise to Improve Insulin Resistance
Eating good foods and avoiding bad foods can help improve insulin resistance or impaired insulin sensitivity, however, there’s another natural way to improve this health issue: exercise. Participating and engaging in regular exercise helps improve insulin resistance or impaired insulin sensitivity associated with prediabetes and type 2 diabetes, among other health issues, by moving sugar from the bloodstream into the muscles to be used for energy. The American Heart Association recommends approximately 150 minutes of exercise every week for adults. Participating or engaging in exercise on a daily basis can improve high blood sugar levels as well as promote overall health and wellness.
For more information regarding how to naturally improve insulin resistance, please review this article:
Nutritional Modulation of Insulin Resistance
Insulin is an essential hormone that is naturally produced in the pancreas to help regulate blood sugar levels and move excess sugar from the bloodstream into the cells to be used for energy. When the pancreas senses high blood sugar levels in the blood, it creates more insulin to help reduce glucose. However, this can decrease the amount of insulin-producing cells in the pancreas which can cause insulin resistance or impaired insulin sensitivity. If the pancreas can’t produce enough insulin, it can ultimately lead to prediabetes or type 2 diabetes, among other health issues. There are several natural ways to improve insulin resistance or impaired insulin sensitivity to prevent and regulate prediabetes and type 2 diabetes, including eating good foods, avoiding bad foods, and exercising. Furthermore, adding a variety of good foods to a smoothie can be a fast and easy way to add nutrients to your diet. – Dr. Alex Jimenez D.C., C.C.S.T. Insights

Sweet and Spicy Juice
Servings: 1
Cook time: 5-10 minutes
- 1 cup honeydew melons
- 3 cups spinach, rinsed
- 3 cups Swiss chard, rinsed
- 1 bunch cilantro (leaves and stems), rinsed
- 1-inch knob of ginger, rinsed, peeled, and chopped
- 2-3 knobs whole turmeric root (optional), rinsed, peeled, and chopped
Juice all ingredients in a high-quality juicer. Best served immediately.

Eat Mushrooms
One simple thing we can do to improve the microbiome!
Mushrooms feed bacteria in the gut. They are rich in chitin, hemicellulose, ? and ?-glucans, mannans, xylans, and galactans. They are also amazing prebiotics that promotes the growth of gut microbiota, equalling health benefits.
The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues or functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or disorders of the musculoskeletal system. Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. To further discuss the subject matter above, please feel free to ask�Dr. Alex Jimenez�or contact us at�915-850-0900.
Curated by Dr. Alex Jimenez D.C., C.C.S.T.
References:
- Raman, Ryan. �14 Natural Ways to Improve Your Insulin Sensitivity.� Healthline, Healthline Media, 17 May 2017, www.healthline.com/nutrition/improve-insulin-sensitivity.
- Herrmann Dierks, Melissa. �Meal Planning & Exercise Tips for Insulin Resistance.� AgaMatrix, AgaMatrix Media, agamatrix.com/blog/insulin-resistance-diet/.
- Felman, Adam. �Diet and Insulin Resistance: Foods to Eat and Diet Tips.� Medical News Today, MediLexicon International, 27 Mar. 2019, www.medicalnewstoday.com/articles/316569#foods-to-eat.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Chiropractic, Diets, Epigenetic's, Functional Medicine, Health, Nutrition, Nutritional Genomics, Supplements, Wellness
Researchers are trying to understand how nutrigenomics can affect a person’s health. Studies have shown that epigenetics increases the risk of several health issues. Other studies have also shown that nutrition can change the risk of disease. For many years, researchers have studied the way that traits in plants and animals are passed down between generations. However, this process is still not well understood. A recent study evaluated how epigenetic marks are passed down between generations of pregnant rats given personalized nutrition. The findings showed both genetic and characteristics changes in the rats’ offspring. This suggests that maternal traits and diet may send different signals to the fetus.
Another study showed methylation changes in mice given more methyl donor intakes over six generations. These findings demonstrated that genetic and characteristic changes passed down between generations may be how environmental factors affect genes in plants and animals to allow adaptation to different environments.�The purpose of the following article is to discuss how nutrigenomics and traits between generations can ultimately affect a person’s overall well-being.
Epigenetics, Nutrition, and Exercise
Researchers have determined that the role of epigenetics in health issues like cancer is caused by methylation changes in several different types of genes and it is commonly associated with aging. However, the increased risk of cancer may be due to factors in the person’s immediate course of life where changes in epigenetics may happen years before the development of health issues like cancer. One study found that methylation of the breast-cancer-related gene is associated with the increased risk of early-onset breast cancer. Other studies have shown that resveratrol prevents methylation changes while folic acid affected gene expression associated with changes in methylation and other functions.
Eicosapentaenoic acid also caused methylation changes in the tumor suppressor gene associated with leukemia cells. This study demonstrated the effect of a polyunsaturated fatty acid on epigenetics. Another study found that methylation increased in women diagnosed with human papillomavirus that didn’t have cervical intraepithelial neoplasia. The changes in methylation were associated with higher concentrations of folate and cobalamin in the blood stream. Another study also found that methylation changes in the tumor suppressor gene L3MBTL1 were ultimately associated with overall health. Further studies are necessary to determine how nutrition can affect epigenetics and traits between generations.
Two studies evaluated the effects of exercise on methylation. One of the studies showed methylation changes in people who participated in physical activities for about 30 minutes every day compared with people who engaged in physical activities for less than 10 minutes every day. In the other study, volunteers who participated in exercise demonstrated changes in methylation and gene expression. These findings suggest that methylation is affected by physical activity.
Nutrigenomics and Risk of Health Issues
Numerous studies have evaluated the role of epigenetics in people with diabetes. According to researchers, changes in methylation of several genes have been shown to be associated with insulin resistance in patients with diabetes. A single change in gene expression caused significant methylation changes in people with diabetes compared to healthy controls. However, other studies found changes in traits between generations and obesity. Furthermore, methylation changes did happen in people with normal glucose metabolism which then developed impaired glucose homeostasis. Various genes have been shown to be different in people with diabetes compared to healthy controls, according to the studies.
According to numerous other studies, twins were found to have increased methylation associated with increased insulin resistance. These findings suggest that epigenetic marks associated with diabetes may occur before symptoms and determine the risk of disease. In conclusion, increasing evidence has demonstrated that nutrition can ultimately cause changes to a person’s epigenetics and how these are associated with the increased risk of developing health issues.
Healthcare professionals and researchers have demonstrated that we can change our epigenetics and gene expression as well as improve the risk of developing a variety of health issues, including inflammation and cancer, which can ultimately cause chronic pain, by controlling the food we eat and focusing on our nutrigenomics. Starting in the kitchen and then taking it directly to the genes, if we follow balanced nutrition, we will see a significant change in our overall health and well-being. At our clinic, we have the ability to assess your specific genetic factors and what dietary guidelines are best for you. One test we use for this is from DNA life, called DNA Diet. A sample of this report is shown below:�
http://www.dnalife.healthcare/wp-content/uploads/2019/06/DNA-Diet-Sample-Report-2019.pdf
Studies show that nutrition can affect methylation and gene expression. These studies have also found that balanced nutrition can improve how good food affects our overall health and well-being. The following article discussed how our epigenetics can affect traits passed down between generations, including methylation and the risk of disease. Although a good diet is essential it may be difficult for some people to follow. Drinking juices or smoothies can be easy ways to include the balanced nutrition we need to promote our health and well-being. Below, I’ve provided a smoothie recipe so you can address your nutrigenomics from the kitchen to your genes. – Dr. Alex Jimenez D.C., C.C.S.T. Insights

Berry Bliss Smoothie
Servings: 1
Cook time: 5-10 minutes
- 1/2 cup blueberries (fresh or frozen, preferably wild)
- 1 medium carrot, roughly chopped
- 1 tablespoon ground flaxseed or chia seed
- 1 tablespoons almonds
- Water (to desired consistency)
- Ice cubes (optional, may omit if using frozen blueberries)Blend all ingredients in a high-speed blender until smooth and creamy. Best served immediately.
The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues or functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or disorders of the musculoskeletal system. Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. To further discuss the subject matter above, please feel free to ask�Dr. Alex Jimenez�or contact us at�915-850-0900.
Curated by Dr. Alex Jimenez D.C., C.C.S.T.
References:
- KA;, Burdge GC;Hoile SP;Lillycrop. �Epigenetics: Are There Implications for Personalised Nutrition?� Current Opinion in Clinical Nutrition and Metabolic Care, U.S. National Library of Medicine, 15 Sept. 2012, pubmed.ncbi.nlm.nih.gov/22878237/.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Chiropractic, Diets, Functional Medicine, Health, Natural Health, Wellness
Healthcare professionals commonly give nutritional recommendations based on an entire population, only sometimes changing these according to age, sex, and pregnancy. Over the last 20 years, however, an increase in research studies has demonstrated that epigenetics can ultimately affect nutrition and even increase the risk of developing a variety of health issues, including oxidative stress and inflammation. Recent advances in technology are also currently being used to help healthcare professionals understand how nutrigenomics can affect an individual’s overall health and wellness.
Several research studies have also demonstrated that single nucleotide polymorphisms can explain the risk for individual complex disease traits. A single-nucleotide polymorphism, or SNP, is a substitution of a nucleotide that happens in the genome. Moreover, further research studies can be utilized to explain the variation in health issue risk based on nutrition and genome. The purpose of the following article is to discuss recent developments in the field of epigenetics and personalized nutrition as well as to consider the contribution of research studies to nutritional recommendations.
Understanding Nutrigenomics
Epigenetics is a collection of changes that affect chromatin structure, without altering our DNA sequence, while allowing transcriptional regulation over a range of timescales. Common epigenetic processes include histone modification, non-coding RNAs, and DNA methylation. Many research studies on how epigenetics affects personalized nutrition focus on DNA methylation, however, research findings have demonstrated other epigenetic marks. DNA methylation inside a dinucleotide is a well-known modification in the genome of a variety of mammals in DNA replication and cell division.
Methylation of dinucleotides is shown by DNA methyltransferases, or Dnmts, and is regulated by mitosis. DNA methylation can trigger transcriptional silencing by blocking and/or promoting the connection of transcription factors in the methyl CpG-binding protein MeCP2 which activates histone-modifying complexes to the DNA. MeCP2 activates what is frequently referred to as histone deacetylases, or HDACs, and histone methyltransferases, or HMTs, resulting in a closed chromatin structure and transcriptional silencing. These have been demonstrated to be associated with various health issues.
Dnmt1 is activated by HDACs and HMTs which suggests that chromatin structure may also affect the status of DNA methylation in the regulation of genes associated with nutrition. According to healthcare professionals, epigenetic marks are essentially maintained throughout an individual’s life. However, recent research findings show that epigenetic plasticity can be affected in early development, including in stages of increased physiological changes, such as puberty and aging. This ultimately suggests the possibility that epigenotypes associated with the increased risk of developing health issues can change.
Epigenetics, Personalized Nutrition, and Origins of Health Issues
Research studies have demonstrated that our early life environment can affect our epigenetic process and the origins of health issues. Healthcare professionals also believe that nutrition in our early life can affect our epigeno- and phenotype in the future. Pregnant rat groups given a diet with corn oil resulted in hypermethylation and decreased gene expression in the offspring, causing mature osteoblasts. This is the first research study to find how maternal diet affects epigenetic processes by altering morphogenesis and changing non-imprinted gene expression in pregnant rat groups.
Pregnant rat groups given a diet with an undisclosed type of fat had increased fetal blood glucose concentration and increased mRNA expression of gluconeogenic genes in the fetal liver. A recent research study found that the amount of fat in the maternal diet was one of the major factors resulting in epigenetic changes in the offspring of pregnant rat groups. The diets given to the pregnant rat groups including fat derived from safflower oil, butter, hydrogenated soybean oil, or fish oil caused hypermethylation in the offspring compared with those where pregnant rat groups were only given 7 percent fat.
Furthermore, one research study found that pregnant rat groups given a protein-restricted, or PR, diet developed epigenetic silencing in both histone modifications and DNA methylation which was followed by progressive transcriptional suppression as the offspring aged. The research findings suggest that nutrition during early development can ultimately cause long-term changes in phenotype. Pregnant rat groups given a PR diet also developed hypomethylation of specific dinucleotides in the adipose tissue leptin promoter and in the heart PPARa promoter in adult offspring.
For more information regarding how epigenetics affects personalized nutrition, please review this article:
Epigenetics: Are There Implications for Personalised Nutrition?
Healthcare professionals have demonstrated that we can change gene expression and improve the risk of developing a variety of health issues, including oxidative stress and inflammation which can cause chronic pain, by controlling the food we eat. Starting in the kitchen and then taking it to the genes, if we follow a balanced nutrition, we will see a considerable change in our overall health and wellness. We have the ability to assess your specific genetic factors and what dietary guidelines are best for you to follow. One test we use is from DNA life called DNA Diet. A sample of this report is shown below:�
DNA-Diet-Sample-Report-2019.pdf
Research studies have demonstrated how epigenetics affect personalized nutrition. The same research studies have also demonstrated that a balanced nutrition can change our gene expression to improve how good food affects our overall health and wellness. While following a proper diet can help improve the risk of developing a variety of health issues, including oxidative stress and inflammation associated with chronic pain, eating good food may be difficult for some people. That’s why drinking smoothies or juices can be easy ways to include the balanced nutrition we need to promote our well-being. In the section below, I’ve provided a smoothie recipe so you can take your health and wellness from the kitchen to your genes. – Dr. Alex Jimenez D.C., C.C.S.T. Insights

Sea Green Smoothie
Servings: 1
Cook time: 5-10 minutes
� 1/2 cup cantaloupe, cubed
� 1/2 banana
� 1 handful of kale or spinach
� 1 handful of Swiss chard
� 1/4 avocado
� 2 teaspoons spirulina powder
� 1 cup water
� 3 or more ice cubes
Blend all ingredients in a high-speed blender until completely smooth and enjoy!
The scope of our information is limited to chiropractic, musculoskeletal, and nervous health issues or functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or disorders of the musculoskeletal system. Our office has made a reasonable attempt to provide supportive citations and has identified the relevant research study or studies supporting our posts. We also make copies of supporting research studies available to the board and or the public upon request. To further discuss the subject matter above, please feel free to ask�Dr. Alex Jimenez�or contact us at�915-850-0900.
Curated by Dr. Alex Jimenez D.C., C.C.S.T.
References:
KA;, Burdge GC;Hoile SP;Lillycrop. �Epigenetics: Are There Implications for Personalised Nutrition?� Current Opinion in Clinical Nutrition and Metabolic Care, U.S. National Library of Medicine, 15 Sept. 2012, pubmed.ncbi.nlm.nih.gov/22878237/.































