
Genetic Testing In Integrative And Functional Medicine
Genetic: Integrative and functional medicine came to the forefront for many medical practitioners and patients alike when they
became dissatisfied with traditional medicine�s sole focus on what was considered �science-based� treatment approaches. Traditional medicine�s viewpoint of dealing with symptoms in isolation from the rest of a patient�s body, mind, and spirit can be too confining when it comes to certain conditions.
This evolution to a more function-centered approach as opposed to a disease-centered way of seeing the whole person has led to improved healthcare. It also looks at prevention, not simply illness and at living in a healthy state, not simply disease-free.
What Is Integrative & Functional Medicine?
Practitioners of integrative and functional medicine take into consideration genetic, environmental, and lifestyle issues when listening to their patients describe the symptoms plaguing them. Their inclusion of these issues makes the process more of a natural medicine approach.
With the dramatic increase in chronic illness conditions and the lack of training traditional physicians have in dealing with these conditions, the move into integrative and functional medicine is needed.
Many of these chronic illness conditions have a genetic component that, along with environmental and lifestyle factors, lead to serious limitations on people�s lives. This shows the importance of the individual biochemical and genetic aspects of each person on his or her health.
This other approach in medicine realizes the necessity of considering nutrition, exercise, diet, and genetics in evaluating and remediating chronic illness conditions. The use of genetic testing in integrative and functional medicine is one way to take all of these factors into account.
SNPs & Integrative & Functional Medicine
Upon completion of the mapping of the human genome, we know there are 20-25,000 genes in each genome. With this knowledge came the information that there are over 80 million variants in the human genome.
These variants are comprised in part of single nucleotide polymorphisms (SNPs) and deletions or insertions in the genome. It is these SNPs that provide significant health information to providers of integrative and functional medicine to prevent or alleviate chronic illness conditions.
Knowing the presence of and placement of SNPs through genetic point mutation testing allows evaluation of the susceptibility to develop many of the chronic illness conditions that affect people today. In addition, this kind of testing helps pinpoint relevant SNPs and their corresponding metabolic markers in individuals.
Testing of this kind provides targeted interventions through the use of traditional medicine approaches as well as supplementation through integrative and functional medicine approaches. Monitoring of individuals� progress is also made easier with genetic testing by measuring metabolic markers found in the original tests over a period of time.
Individual monitoring of this type is necessary when this kind of personalized intervention and supplementation is used. If there is an overload of either medications or supplementations, there can be an impact on the performance of metabolic processes that can lead to side effects. These side effects can influence functions and responses, such as the immune response.
Individual SNPs will determine how well medications and supplements are working.
Genetic Testing In Relation To Diet & Weight Loss
Integrative and functional medicine practitioners not only deal with illness, they also provide health and wellness evaluations. Current research has shown how important a role genetics plays in the prevention of many chronic health conditions.
Genetic testing can show vulnerabilities to conditions and suggest options for individuals. This kind of testing can also provide valuable information concerning how individuals can respond to different attempts to live more healthy lives.
Genetic testing has been shown to be effective in several areas: diet, eating behavior traits, nutritional needs, exercise, body and weight, and metabolic health. For each of these areas, there are certain genetic markers that can provide information regarding how genetics will affect each of these areas.
Diet
 People are seemingly obsessed with weight. How to lose it and keep it off, how to re-distribute it to look more attractive. Professionals in integrative and functional medicine are approached regularly for help in this area.
People are seemingly obsessed with weight. How to lose it and keep it off, how to re-distribute it to look more attractive. Professionals in integrative and functional medicine are approached regularly for help in this area.
Everyone knows it�s hard for some people to lose weight on any kind of diet, while others can lose weight any time they want. It�s not just due to lack of willpower that people don�t lose the weight they want. It may also be due to genetics.
Research has shown about 88 percent of people have bodies that resist burning fat through low-intensity exercise. Most people will gain weight if they eat almost any carbs (about 45 percent of people) or almost any fat (about 39 percent of people).
The reason for this is a diet and type of exercise matched to specific genotype lead to weight loss. These diets and exercise types are not the same for everyone.
For example, let�s look at adrenoceptor Beta 3 (ADRB3) with an SNP on rs4994. There are different variations of this gene. If you are either an AA or TT genotype, you have what is called a genetic privilege and just about any kind of exercise will work for you. On the other hand, if you don�t have either of these AA or TT genotypes, this is a genetic disprivilege and only a high-intensity type exercise will help you lose weight.
Further analysis of other genes and SNPs can tell you the type of diet, either low carb or low fat, that will work best for you. In fact, using a diet matched to your genetics can result in a loss of two and half times as much weight as a diet not matched to genetics.
In addition to choosing the right diet to lose weight, choosing the right diet may also help you avoid developing a chronic health condition. Research has shown diet to be implicated in many chronic illness conditions, so genetic testing to determine your specific vulnerability to illnesses and your response to particular foods may help prevent them.
Knowing your predisposition to illnesses can lead to targeted dietary and lifestyle changes that may modify any existing conditions and help prevent future developments. Future research may bring more information regarding bioavailable components in foods that can aid in alleviating health issues.
COMT & CYP19 Genes
 Research has identified certain genes that work together and appear to show that some people retain fat regardless of, or in spite of, exercise.
Research has identified certain genes that work together and appear to show that some people retain fat regardless of, or in spite of, exercise.
In one study, researchers found two genes, COMT and CYP19 that appeared to be involved in patterns of fat loss and exercise. Having one CYP19 gene and variants of that gene did not affect fat, intra-abdominal fat, or total fat. However, having two of these genes seemed to be related to slightly more decrease in body mass index and significantly more decrease in total fat and percentage of body fat.
The researchers also found that having one genotype of the COMT gene and one copy of the CYP19 gene seemed related to significant loss of BMI, total fat, and percentage of body fat.
Why and how these genes and combinations work isn�t known yet. More research is needed to determine this. Other research suggests women with a specific CYP19 variant may also have increased levels of estradiol and estrone which may make it harder for them to lose fat through exercise.
Environmental Factors
Weight loss or gain is not solely at the mercy of your genetics however. A combination of genetics and environment is likely behind your success or failure regarding your weight loss attempts.
The thinking of professionals is divided on the subject of genetics versus environment/lifestyle choices. One set of these professionals regards environment to be the telling component. They point to the teaching over the years that food is a reward for good performance at anything. This, combined with constant reminders about food that are around us all the time, makes it hard for some people to lose weight and/or keep it off.
Others believe losing weight and keeping it off are more related to biological functions. They have found people to be metabolically different after losing up to ten percent of their body weight. Their brains also seem to respond to food differently. The emotional response to food is greater, but the brain regions that deal with food restraint are less active. This sets up the person to regain the weight lost.
Further research into why people lose weight and maintain that loss will be needed. Some of that research has to be on the genetic basis of weight loss.
Eating Behavior
 Integrative and functional medicine practitioners view eating behavior as important for overall health.�These behaviors include snacking behavior, feelings of satiety, craving for sweets, desire for food or certain foods, and the disinhibition of eating.
Integrative and functional medicine practitioners view eating behavior as important for overall health.�These behaviors include snacking behavior, feelings of satiety, craving for sweets, desire for food or certain foods, and the disinhibition of eating.
Nutrigenetics and nutrigenomics are two new fields of study related to how genes affect our diet and how our diet affects genes, respectively. Obesity, cancer, and heart disease are three of the health conditions most investigated in these two new fields.
One study involving these new fields showed the bitter taste gene receptor hTAS2R38 to be involved in tasting glucosinolates, found in some fruits and vegetables. Three genotypes in this gene receptor have been identified: PAV/PAV, PAV/AVI, and AVI/AVI.
Those individuals with PAV/PAV are said to be supertasters. They are very sensitive to bitter tastes in some foods and in some man-made compounds used in research. People with PAV/AVI are considered medium tasters. They can taste bitter in the research compounds, but not as much as the supertasters. Individuals with AVI/AVI are labeled non-tasters. They don�t taste bitter in the research compounds.
While it�s difficult to completely understand why these differences occur, it does appear they can make a difference in people�s diets. It could be that people who taste bitter greatly or somewhat will avoid certain vegetables that contain this bitter taste. Vegetables like kale and broccoli have this taste.
In this way, genetics have a significant influence on eating behavior.
 Research indicates taste is only one of the ways genetics affects eating behavior. Caloric intake, meal size, and frequency of eating also appear to be affected. People�s desire for fats, carbohydrates, or proteins also may be influenced by genetics.
Research indicates taste is only one of the ways genetics affects eating behavior. Caloric intake, meal size, and frequency of eating also appear to be affected. People�s desire for fats, carbohydrates, or proteins also may be influenced by genetics.
Research has found apolipoprotein A-II (APOA2) to be implicated in this kind of desire. Three variants in this gene, TT, TC, and CC, have been isolated as factors affecting the choice of fats, carbs, and proteins. One study showed both men and women who had the recessive CC chose more fat and protein and fewer carbs than either of the T alleles. The CC group ate about 200 more calories than the other group and tended to develop obesity more frequently.
It appears that APOA2 may affect not only food choices but also feelings of satiety.
Nontasters seem to prefer and seek out fats and flavors, so dieting may be more difficult for them to stick with and lose weight. Supertasters, on the other hand, enjoy a variety of foods, especially those that are spicy and robust. This may help them with diets.
Understanding the factors that appear to influence eating behaviors has gained importance with the tremendous increase in obesity in the U.S. and around the world, along with diabetes and cardiovascular disease. Eating behavior must be seen as a complex inter-relationship among psychological, cultural, physical, and genetic factors that influence the choice of foods, the amount of food intake, caloric intake, and timing of meals.
Regulating Eating Behavior
Clearly, taste affects food choices as seen in the discussion above. Another of the bitter receptors, TAS2R5, may also assist in regulating eating behavior. Alcohol dependence has been associated with an SNP in this receptor, along with another receptor, TAS2R16. These research findings seem to indicate variants in the TAS2R gene to be associated with ingestive behavior.
Genetic influence over meal amounts, how often people eat, and the timing of meals is a new area of study and may involve digestive neuroendocrine hormones such as CCK, leptin, and ghrelin. Studies are underway investigating the effects of these hormones on pathways that influence eating behavior.
A gene with a strong association with the risk of obesity, FTO, appears to contribute to obesity by downregulating leptin production in adipocytes. Adiposity and satiety appear to be associated with a fairly common variant, rs9939609. One study showed the A allele of rs9939609 to influence post-meal feelings of satiety and possibly to influence the excess caloric intake seen in men and women with high BMIs.
A gene involved in the detoxification of nutrients during digestion, AKR1B10, also appears to play a role in influencing human eating behavior.
Nutritional Needs & Genetic Testing
Another area in which integrative and functional medicine practitioners use genetic testing is in�determining nutritional needs of their patients. As we have seen previously, genetic variants have an effect on taste and thus on nutrition. When people choose foods that �fit� their tastes but are short on nutrients, their health suffers. People also appear to have genetic responses to some supplements, such as some of the B vitamins and vitamin C.
The impact of nutrition is a lifetime factor, and practitioners of integrative and functional medicine evaluate nutritional needs closely. Any genetic variant that leads to abnormal nutritional requirements would likely be incompatible with survival. For example, miscarriage is more likely in a woman whose fetus has two alleles that negatively affect the use of any given nutrient than a woman whose fetus just has the common functional variants.
Several studies have isolated genes and alleles that affect nutrients and their utilization. For example, an SNP (Ala222Val) in the methylenetetrahydrofolate reductase (MTHFR) gene leads to a significant alteration in folate metabolism, increasing the risk of neural tube defects (NTDs) and cardiovascular disease, but lowering the risk of colon cancer. Increasing folate intake lowers the risks of developing serious health conditions.
Research has found other SNPs that alter homocysteine metabolism and folate uptake and transport. SNPs in enzymes that affect utilization and metabolism of vitamin B12 seem to be associated with NTDs and the possible development of Down syndrome and colon cancer.
SNPs in the vitamin D receptor may be associated with asthma in both children and adults. Lipid pathways, alcohol metabolism, and lactose metabolism appear to be affected by SNPs in other genes, also. A beneficial effect of these SNPs in the ancestors of certain ethnic groups or ancestral subpopulations may have been present, even though they tend to carry the risk of an adverse outcome today.
Environmental changes have been shown to bring a previously silent allele into a role as a disease allele. The aldolase B enzyme metabolizes fructose and was silent even with a high number of polymorphisms. In recent times, when fructose was added to foods as a sweetener, the polymorphisms began presenting as disease alleles.
Integrative and functional medicine professionals can use this information to guide their patients into more healthy lives.
Genetic Testing & Exercise
 Integrative and functional medicine also uses genetic testing to determine the best types of exercise for different people and to explore the likelihood of injuries of several kinds in athletes. This latter area of research and clinical practice can help reduce the number and severity of athletic injuries for adult and child athletes.
Integrative and functional medicine also uses genetic testing to determine the best types of exercise for different people and to explore the likelihood of injuries of several kinds in athletes. This latter area of research and clinical practice can help reduce the number and severity of athletic injuries for adult and child athletes.
While there have been some gene variants associated with athletic ability, none have been shown to be predictive to any degree. Research in this area is promising for decreasing serious injury in young athletes. But to date, little scientific information regarding a genetic variation in young athletes is available.
Genetic testing as a way of choosing which athlete to select for a particular sport is increasing. However, little evidence has been found to show it is more accurate than traditional ways of selecting candidates. The ethics of this kind of testing for young athletes has been brought into question.
ACE Genes
Two genes and the SNPs associated with them have been examined in several population samples and thus have robust findings. The ACE I/D polymorphism was first found to be associated with human performance several years ago. This gene is part of the renin-angiotensin system that controls blood pressure through its effect on the regulation of body fluid levels.
The ACE I allele lowers ACE activity in serum and tissue. The D allele increases ACE activity in serum and tissue. The ACE I/I genotype has been shown over and over again to indicate performance endurance and greater efficiency in exercise. The ACE DD genotype has been shown to indicate strength and power performance levels.
This ACE I/D genotype does not appear to have predictive ability in Kenyan athletes, suggesting the confounding influence of ethnicity or geography.
ACTN3 Gene
 The ACTN3 is strongly associated with the protein alpha-actinin-3. This protein is involved exclusively in fast type II muscle fibers that are used in explosive activities. SNP R577X indicates a stop codon at position 577 rather than an arginine (R). An R allele puts athletes at an advantage in power sports. A study of the ACTN3 R577X variant in elite European athletes showed those in power event to be 50 percent less likely to have the XX variant and those involved in endurance events to be 1.88 times more likely to have the XX variant. For world-class endurance athletes, the odds of having the XX variant were 3.7 times larger when compared with lower-level athletes. It appears the ACTN3 gene is more important at the upper levels of sports.
The ACTN3 is strongly associated with the protein alpha-actinin-3. This protein is involved exclusively in fast type II muscle fibers that are used in explosive activities. SNP R577X indicates a stop codon at position 577 rather than an arginine (R). An R allele puts athletes at an advantage in power sports. A study of the ACTN3 R577X variant in elite European athletes showed those in power event to be 50 percent less likely to have the XX variant and those involved in endurance events to be 1.88 times more likely to have the XX variant. For world-class endurance athletes, the odds of having the XX variant were 3.7 times larger when compared with lower-level athletes. It appears the ACTN3 gene is more important at the upper levels of sports.
While research shows the effects of the ACTN3 gene on athletic performance, especially in higher class athletes, the effects in the general population were negligible. It is unclear just what the association of this gene in the general population and choice of athletic activities in this population might be.
Resistance to injury and the ability to recover from injuries are also very important factors not only in professional sports but also for the general population. The emphasis on physical activity currently seen in the culture increases the risk of injury and the need for information regarding recovery.
Concussions and tendinopathies have been studied fairly extensively. Information on these two growing areas of injury among young athletes has been valuable for integrative and functional medicine specialists.
These two areas are important due to the long-lasting effects of both on young athletes. Research and clinical practice have shown the effects of concussion to linger into old age where they can increase the cognitive decline normally seen at that time of life.
APOE4 Gene
A better understanding of the genetic aspects of injury and recovery can help practitioners of integrative and functional medicine to both protect those young athletes at risk for injury and to better treat those who suffer injuries.
Regarding concussion, the gene most studied is APOE and its three alleles. The APOE e4 allele has been implicated in the development of Alzheimer�s Disease. This allele has been studied recently to determine its association, if any, with concussion risk and outcomes of traumatic brain injury. To date, the results are not clear.
Some findings have shown people with the e4 allele to have less favorable outcomes from traumatic brain injuries and boxers with this allele had higher chronic brain injury scores. These findings are consistent with e4 being a risk allele. However, one study of college athletes with the e4 allele did not find them to be more likely to suffer a concussion. Another study showed the e4 allele was not associated with poorer head trauma outcomes in children.
Another APOE variant, G-219T, has been linked with increased risk of concussion in athletes. Those athletes with the TT genotype compared to those with the GG genotype had a risk of concussion three times larger. A weak association was found in that same study between the tSer53Pro polymorphism in MAPT, the tau-protein encoding gene, and risk of concussion.
Collagen Genes, Integrative &Functional Medicine
Collagen is the primary component of tendons and ligaments, thus it is connected very closely with research into tendinopathies. It is no surprise that two variants in genes coding for collagen (COL1A1 and COL5A1) have been shown to suggest increased risk of injury to tendons. MMP3, a gene associated with connective tissue wound repair and the gene encoding TNC, an extracellular matrix protein, have also been implicated in increased risk of tendinopathies.
These are preliminary studies that need replication and further study to validate the findings.
Genetic Testing & Metabolic Health
 Metabolic syndrome and metabolic health have been studied extensively due to metabolic syndrome being a major risk factor for the development of diabetes mellitus 1 and cardiovascular disease. Genetic and environmental factors interrelate in a complex fashion to bring about this condition. A cluster of metabolic abnormalities, including hypertension, dyslipidemia, abdominal obesity, insulin resistance, and impaired glucose tolerance make up metabolic syndrome.
Metabolic syndrome and metabolic health have been studied extensively due to metabolic syndrome being a major risk factor for the development of diabetes mellitus 1 and cardiovascular disease. Genetic and environmental factors interrelate in a complex fashion to bring about this condition. A cluster of metabolic abnormalities, including hypertension, dyslipidemia, abdominal obesity, insulin resistance, and impaired glucose tolerance make up metabolic syndrome.
All of the components of metabolic syndrome are highly heritable. Studies have shown links between metabolic syndrome and genes such as PPARg, adiponectin, CD36, and beta receptors.
There has been a considerable investigation into the heritability of metabolic syndrome. One study involved over 2,200 individuals in over 500 family groups. It was the first to identify major genes influencing metabolic syndrome.
Chromosome 3q27 was significantly linked to six factors involved in metabolic syndrome: weight, leptin, insulin, waist circumference, hip circumference, and insulin/glucose ratio. Chromosome 17p12 was strongly linked to plasma leptin levels.
Another study evaluated over 200 SNPs in 110 genes for their effects on coronary artery disease, highly implicated in metabolic syndrome. SNPs in eight of these genes showed association with metabolic syndrome: LDLR, GBE1, IL1R1, TGFB1, IL6, COL5A2, SELE and LIPC.
These genes are described below:
- LDLR: Low Density Lipoprotein Receptor gene. It is strongly involved in the homeostasis of cholesterol. Hypercholesterolemia in families has been linked to mutations of this gene.
- GBE1: Glycogen Branching Enzyme gene. It is involved in coding the glycogen branching enzyme which aids in glycogen synthesis. Branching of these chains allows a great number of glycosyl units to be stored in a molecule of glycogen.
- IL1R1: Interleukin 1 Receptor, Type 1. Interleukin 1 is made up of two proteins, IL1-alpha and IL1-beta, and is a mediator of inflammation.
- TGFB1: Transforming Growth Factor, Beta 1. This gene encodes the peptide involved in many functions in cells. Apoptosis may result due to dysregulation of the activation of this gene.
- IL6: Interleukin 6 gene. It is a cytokine that regulates the immune response by activating a cell surface signaling assembly. Its production by neoplastic cells has been implicated in the growth of a number of cancers.
- COL5A2: Collagen, Type V, Alpha 2. Mutations in the gene may bring on weakened connective tissue throughout the body.
SELE: Selectin E gene. May be involved in the pathogenesis of atherosclerosis.
Some of the more common inherited metabolic conditions include:
- Lysosomal storage disorders. These can result in the buildup of toxic substances inside lysosomes in the cells.
- Glycogen storage conditions. Sugar storage problems can lead to weakness, low blood sugar, and muscle pain.
- Mitochondrial disorders: Can lead to muscle damage.
- Peroxisomal disorders: Can lead to a buildup of toxic products of metabolism.
- Metal metabolism disorders: Special proteins control levels of trace metals in the blood. A malfunction in these proteins caused by genetic metabolism disorders can lead to toxic levels of metals in the body.
Symptoms of genetic metabolism disorders include:
- Low energy levels
- Decreased appetite
- Abdominal pain
- Weight loss
- Jaundice
- Seizures
From this list of symptoms, it�s easy to see the relationship�of metabolic syndrome and adrenal fatigue. Practitioners of integrative and functional medicine will be faced with patients who present with adrenal fatigue and these similar symptoms. This makes it important for them to understand at least the basics behind Adrenal Fatigue Syndrome (AFS).
Adrenal Fatigue Syndrome
 Feelings of fatigue and lethargy are presented more and more frequently in health care professionals� offices. Combined with concentration difficulties, sleep problems, inability to lose weight, feeling your brain is in a fog, fatigue, and lethargy may point to AFS as the basic issue.
Feelings of fatigue and lethargy are presented more and more frequently in health care professionals� offices. Combined with concentration difficulties, sleep problems, inability to lose weight, feeling your brain is in a fog, fatigue, and lethargy may point to AFS as the basic issue.
AFS is a constellation of many nonspecific symptoms that can become debilitating. The onset of the symptoms is slow and can be missed by traditionally trained professionals.
The symptoms of AFS result from�the body�s normal response to stress�from any source. The hypothalamic-pituitary-adrenal (HPA) axis is set into motion, releasing hormones and other chemicals that are designed to deal with stress. At the end of the axis are the adrenal glands that secrete cortisol, the stress fighting hormone. The purpose of this hormone is to limit the effects of stress on the body.
In normal circumstances, once the stress ceases, the cortisol levels decline and the adrenals get a chance to recover. However, in our stress-filled culture, the stresses continue. This puts the demand on the adrenals at an extreme level. At some point, the adrenals are no longer able to secrete cortisol, which results in damage to the body from the effects of stress.
Levels of inflammation and an increased immune response results. Inflammation has been implicated in many chronic illness conditions. It is at this point that the body begins breaking down from the accumulation of symptoms such as fatigue, brain fog, insulin resistance, and increasing inflammation.
NeuroEndoMetabolic (NEM) Response
The traditional medical viewpoint of addressing individual symptoms and/or organs when working to alleviate illness conditions is simply too mechanistic. A more comprehensive viewpoint is needed in order to effectively deal with symptoms of AFS. The NEM model is such a viewpoint.
The model says it is important to consider organ systems operating in an interrelationship in which whatever affects one organ system affects others as well. In this regard, it is in line with�the integrative and functional medicine viewpoint.
The NEM model is a functional approach that looks at interactions between the individual�s environment and the gastrointestinal, endocrine, and metabolic organ systems, among others. This allows a healthcare practitioner to find the root causes, triggers, immediate causes, and genetic factors involved in a person�s illness condition.
This is a much more comprehensive approach to alleviating people�s symptoms and illness conditions.
 Increasing and unrelenting stress is a part of our culture that is detrimental to the health of every individual. The metabolic component of the NEM model added to the neuroendocrine aspect helps professionals to see how localized organ-specific responses and systemic responses are necessary for successfully dealing with stress.
Increasing and unrelenting stress is a part of our culture that is detrimental to the health of every individual. The metabolic component of the NEM model added to the neuroendocrine aspect helps professionals to see how localized organ-specific responses and systemic responses are necessary for successfully dealing with stress.
The metabolic component of our stress response is very subtle in the early stages. But the derangements of our metabolism worsen as time goes on and stress doesn�t stop. By the time the stress response reaches stage 3 or 4, these derangements can become debilitating. At the severe stage, they can lead to hypersensitivity to supplements and to paradoxical reactions.
Very significant and debilitating symptoms begin arising. Often, these lead the person to be bed-ridden due to their severity.
AFS & Genetics
A question integrative and functional medicine experts and those who suffer from AFS all want to know is: Can you inherit AFS?
Before answering that question, you need to understand even if you have a gene or several genes that are involved in a health condition like AFS, it doesn�t mean you will automatically get that condition. Before genes can do anything, either positive or negative, to your health, they have to get the signal to �switch on.�
One good thing about that signal is you have quite a bit of control over it. Scientists and researchers have discovered environment, choices you can make, exert significant control over whether genes are turned on or off. This is called gene expression.
Can you choose to switch specific genes on or off? That�s beyond us at this point. What you can do is make good lifestyle choices, good exercise choices, good diet choices and either activate or de-activate genes in this way. Genetic testing as seen in integrative and functional medicine practices is a way to determine your choices in many areas. Which diet works best for you and what exercises will best benefit you can be answered through this kind of testing.
Answering the specific question posed above, �Can you inherit AFS?�, is a complicated process.
Two genes with significant involvement in this answer are MTHFR and COMT. Both are involved with methylfolate. People with mutations in MTHFR don�t have enough methylfolate leading to less adrenaline because of interference in the methylation process. Methylation aids in the production of adrenaline and other hormones.
The other gene, COMT, is involved in the production of hormones and chemicals in the body. Low levels of methylfolate with this gene leads to lower levels of epinephrine and higher levels of norepinephrine.
The lack of methylfolate with both of these genes, especially MTHFR, leads to feelings of fatigue.
When your body is stricken by stress, both your adrenals and MTHFR are affected. This leads to the fatigue felt by those of you who suffer from AFS. The enzyme that produces dopamine and serotonin is also dependent on methylation to work right. Low levels of methylfolate can lead to low levels of both of these neurochemicals which can then lead to low energy and fatigue.
What Can You Do To Improve Energy Levels?
There are some things you can do to aid in increasing energy and improving the work of the two genes mentioned, MTHFR and COMT.
Balance your blood sugar levels by eating three or four small meals per day. These meals should include good grains like quinoa or rice, good carbs, and vegetables. You can add protein from fish or free-range chicken.
Supplements can help support your adrenal glands and the methylation process also. Vitamin B1, B2, and B6 will help. There are usually no side effects from vitamin B1, but if you should begin feeling any itching, notice any rashes, or have trouble breathing, contact your healthcare professional immediately.
Side effects from B2 are also rare. Very yellow urine will be seen, but this is not serious. If you do have any rashes, breathing trouble, or itching, contact your physician at once.
Taken in large doses for a long time, B6 can cause side effects. Headache, nausea, and drowsiness are enough to contact your healthcare professional at once.
Some people try taking methylfolate (5-MTHF), but this is a labor-intensive effort and could bring on some serious side effects if your body is not ready for it. If your body gets overwhelmed by the 5-MTHF, you can feel headaches, irritability, anxiety, and heart palpitations. Get medical help right away for these side effects.
Despite advance testing, it is important to remember that tests are simply data points of alert. A clinical decision should be made after a detailed consideration of the history and state of the body. A shotgun approach to treating abnormal laboratory values is a common clinical mistake and can lead to negative clinical outcomes.
Conclusion
 The mapping of the human genome has provided an opportunity for researchers and clinicians alike to consider the roles genes play in health and wellness. Discovering the presence and effects of single nucleotide polymorphisms (SNPs) has increased not only our knowledge of how genes affect health, but also has given us tools to use in preventing and remediating many chronic illness conditions.
The mapping of the human genome has provided an opportunity for researchers and clinicians alike to consider the roles genes play in health and wellness. Discovering the presence and effects of single nucleotide polymorphisms (SNPs) has increased not only our knowledge of how genes affect health, but also has given us tools to use in preventing and remediating many chronic illness conditions.
Integrative and functional medicine practitioners have been among the professionals to use this information in a practical sense. Whether AFS can be inherited is yet to be seen. Clinically, we do see a strong correlation from one generation to the next.
Genetic testing to examine the working of MTHFR and COMT may be of some help. Diet and supplements can also increase your chances of these two genes working correctly and alleviating some of the symptoms of AFS.
Because genetic testing is still in the very early phase of development, it is important to take all data points with the right perspective and refrain from treating abnormal laboratory numbers while the root cause of the problem can be masked.
� Copyright 2017 Michael Lam, M.D. All Rights Reserved.









 Joint pain of unknown origin can cause a myriad of debilitating problems, including the additional stress of trying to find effective remedies for joint pain. It can be a scary and confusing time, especially when test results show no abnormalities and your doctor can�t figure out what�s wrong. It�s important to find and address the cause of the inflammation. If you experience other concurring symptoms similar to those of Adrenal Fatigue, find a practitioner who can support your NEM stress response. Proper restorative strategies will help your body cope with both the stress and the
Joint pain of unknown origin can cause a myriad of debilitating problems, including the additional stress of trying to find effective remedies for joint pain. It can be a scary and confusing time, especially when test results show no abnormalities and your doctor can�t figure out what�s wrong. It�s important to find and address the cause of the inflammation. If you experience other concurring symptoms similar to those of Adrenal Fatigue, find a practitioner who can support your NEM stress response. Proper restorative strategies will help your body cope with both the stress and the 

 Rhetoric from the United States government since the early 1990s proclaims that GM foods are no different from their natural counterparts that have existed for centuries. The Food and Drug Administration (FDA) has labeled them �Generally Recognized as Safe,� or GRAS. This status allows a product to be commercialized without any additional testing. According to US law, to be considered GRAS the substance must be the subject of a substantial amount of peer-reviewed published studies (or equivalent) and there must be overwhelming consensus among the scientific community that the product is safe. GM foods had neither. Nonetheless, in a precedent-setting move in 1992 that some experts contend was illegal, the FDA declared that GM crops are GRAS as long as their producers say they are. Thus, the FDA does not require any safety evaluations or labeling of GMOs. A company can even introduce a GM food to the market without telling the agency.
Rhetoric from the United States government since the early 1990s proclaims that GM foods are no different from their natural counterparts that have existed for centuries. The Food and Drug Administration (FDA) has labeled them �Generally Recognized as Safe,� or GRAS. This status allows a product to be commercialized without any additional testing. According to US law, to be considered GRAS the substance must be the subject of a substantial amount of peer-reviewed published studies (or equivalent) and there must be overwhelming consensus among the scientific community that the product is safe. GM foods had neither. Nonetheless, in a precedent-setting move in 1992 that some experts contend was illegal, the FDA declared that GM crops are GRAS as long as their producers say they are. Thus, the FDA does not require any safety evaluations or labeling of GMOs. A company can even introduce a GM food to the market without telling the agency. Taylor�s GMO policy needed to create the impression that unintended effects from GM crops were not an issue. Otherwise their GRAS status would be undermined and they would need the extensive testing and labels that are normally required for food additives. But internal memos made public from a lawsuit showed that the overwhelming consensus among the agency scientists was that GM crops can have unpredictable, hard-to-detect side effects. Various departments and experts spelled these out in detail, listing allergies, toxins, nutritional effects, and new diseases as potential dangers. They urged superiors to require long-term safety studies.5 In spite of the warnings, according to public interest attorney Steven Druker who studied the FDA�s internal files, �References to the unintended negative effects of bioengineering were progressively deleted from drafts of the policy statement (over the protests of agency scientists).�6
Taylor�s GMO policy needed to create the impression that unintended effects from GM crops were not an issue. Otherwise their GRAS status would be undermined and they would need the extensive testing and labels that are normally required for food additives. But internal memos made public from a lawsuit showed that the overwhelming consensus among the agency scientists was that GM crops can have unpredictable, hard-to-detect side effects. Various departments and experts spelled these out in detail, listing allergies, toxins, nutritional effects, and new diseases as potential dangers. They urged superiors to require long-term safety studies.5 In spite of the warnings, according to public interest attorney Steven Druker who studied the FDA�s internal files, �References to the unintended negative effects of bioengineering were progressively deleted from drafts of the policy statement (over the protests of agency scientists).�6 There are several reasons why GM plants present unique dangers. The first is that the process of genetic engineering itself creates unpredicted alterations, irrespective of which gene is transferred. The gene insertion process, for example, is accomplished by either shooting genes from a �gene gun� into a plate of cells, or using bacteria to infect the cell with foreign DNA. Both create mutations in and around the insertion site and elsewhere.13 The �transformed� cell is then cloned into a plant through a process called tissue culture, which results in additional hundreds or thousands of mutations throughout the plants� genome. In the end, the GM plant�s DNA can be a staggering 2-4% different from its natural parent.14 Native genes can be mutated, deleted, or permanently turned on or off. In addition, the insertion process causes holistic and not-well-understood changes among large numbers of native genes. One study revealed that up to 5% of the natural genes altered their levels of protein expression as a result of a single insertion.
There are several reasons why GM plants present unique dangers. The first is that the process of genetic engineering itself creates unpredicted alterations, irrespective of which gene is transferred. The gene insertion process, for example, is accomplished by either shooting genes from a �gene gun� into a plate of cells, or using bacteria to infect the cell with foreign DNA. Both create mutations in and around the insertion site and elsewhere.13 The �transformed� cell is then cloned into a plant through a process called tissue culture, which results in additional hundreds or thousands of mutations throughout the plants� genome. In the end, the GM plant�s DNA can be a staggering 2-4% different from its natural parent.14 Native genes can be mutated, deleted, or permanently turned on or off. In addition, the insertion process causes holistic and not-well-understood changes among large numbers of native genes. One study revealed that up to 5% of the natural genes altered their levels of protein expression as a result of a single insertion. The very first crop submitted to the FDA�s voluntary consultation process, the FlavrSavr tomato, showed evidence of toxins. Out of 20 female rats fed the GM tomato, 7 developed stomach lesions.21 The director of FDA�s Office of Special Research Skills wrote that the tomatoes did not demonstrate a �reasonable certainty of no harm,�22 which is their normal standard of safety. The Additives Evaluation Branch agreed that �unresolved questions still remain.�23 The political appointees, however, did not require that the tomato be withdrawn.1
The very first crop submitted to the FDA�s voluntary consultation process, the FlavrSavr tomato, showed evidence of toxins. Out of 20 female rats fed the GM tomato, 7 developed stomach lesions.21 The director of FDA�s Office of Special Research Skills wrote that the tomatoes did not demonstrate a �reasonable certainty of no harm,�22 which is their normal standard of safety. The Additives Evaluation Branch agreed that �unresolved questions still remain.�23 The political appointees, however, did not require that the tomato be withdrawn.1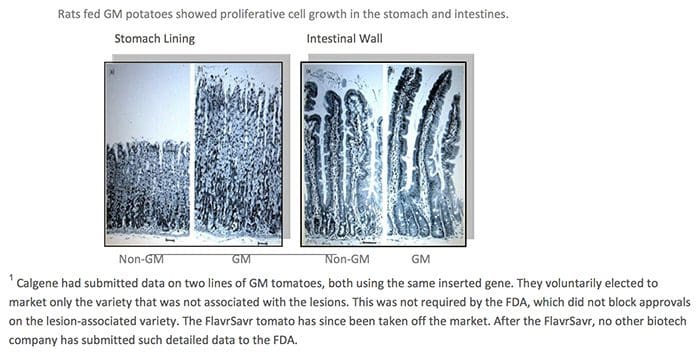 GM Diets Cause Liver Damage
GM Diets Cause Liver Damage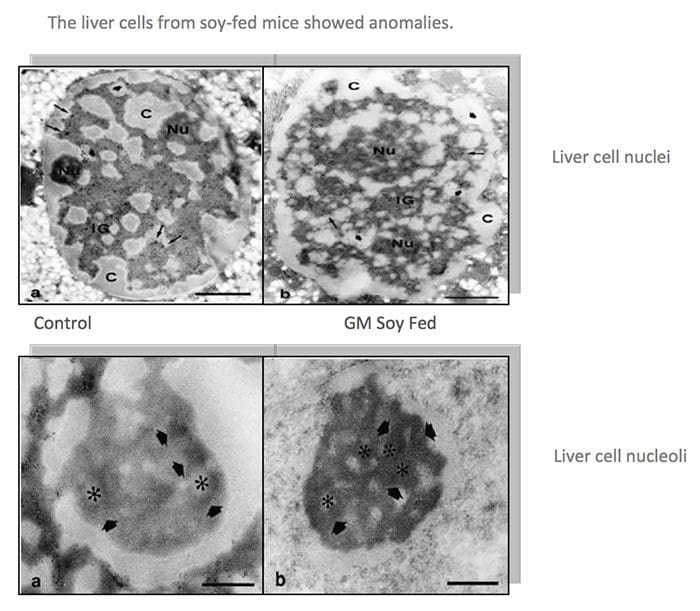
 GM Fed Animals Had Higher Death Rates & Organ Damage
GM Fed Animals Had Higher Death Rates & Organ Damage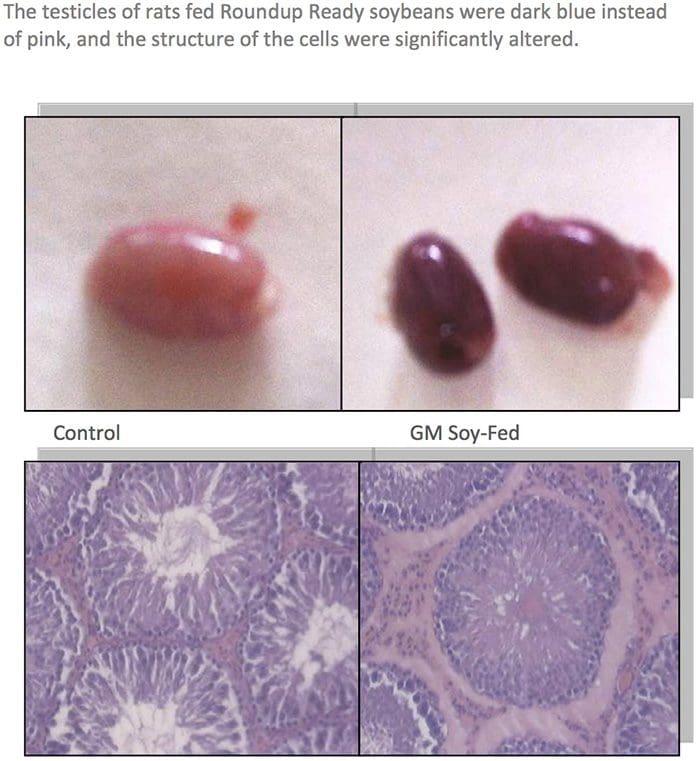

 About two dozen farmers reported that their pigs had reproductive problems when fed certain varieties of Bt corn. Pigs were sterile, had false pregnancies, or gave birth to bags of water. Cows and bulls also became sterile. Bt corn was also implicated by farmers in the deaths of cows, horses, water buffaloes, and chickens.55
About two dozen farmers reported that their pigs had reproductive problems when fed certain varieties of Bt corn. Pigs were sterile, had false pregnancies, or gave birth to bags of water. Cows and bulls also became sterile. Bt corn was also implicated by farmers in the deaths of cows, horses, water buffaloes, and chickens.55 Allergic reactions occur when the immune system interprets something as foreign, different, and offensive, and reacts accordingly. All GM foods, by definition, have something foreign and different. And several studies show that they provoke reactions. Rats fed Monsanto�s GM corn, for example, had a significant increase in blood cells related to the immune system.58 GM potatoes caused the immune system of rats to respond more slowly.59 And GM peas provoked an inflammatory response in mice, suggesting that it might cause deadly allergic reactions in people.60
Allergic reactions occur when the immune system interprets something as foreign, different, and offensive, and reacts accordingly. All GM foods, by definition, have something foreign and different. And several studies show that they provoke reactions. Rats fed Monsanto�s GM corn, for example, had a significant increase in blood cells related to the immune system.58 GM potatoes caused the immune system of rats to respond more slowly.59 And GM peas provoked an inflammatory response in mice, suggesting that it might cause deadly allergic reactions in people.60 For years, organic farmers and others have sprayed crops with solutions containing natural Bt bacteria as a method of insect control. The toxin creates holes in their stomach and kills them. Genetic engineers take the gene that produces the toxin in bacteria and insert it into the DNA of crops so that the plant does the work, not the farmer. The fact that we consume that toxic pesticide in every bite of Bt corn is hardly appetizing.
For years, organic farmers and others have sprayed crops with solutions containing natural Bt bacteria as a method of insect control. The toxin creates holes in their stomach and kills them. Genetic engineers take the gene that produces the toxin in bacteria and insert it into the DNA of crops so that the plant does the work, not the farmer. The fact that we consume that toxic pesticide in every bite of Bt corn is hardly appetizing. Although the number of safety studies on GM foods is quite small, it has validated the concerns expressed by FDA scientists and others. Unfortunately, government safety assessments worldwide are not competent to even identify most of the potential health problems described above, let alone protect its citizens from the effects.88
Although the number of safety studies on GM foods is quite small, it has validated the concerns expressed by FDA scientists and others. Unfortunately, government safety assessments worldwide are not competent to even identify most of the potential health problems described above, let alone protect its citizens from the effects.88 Submissions to the US Food and Drug Administraion (FDA) may be worse than in other countries, since the agency doesn�t actually require any data. Their policy says that biotech companies can determine if their own foods are safe. Anything submitted is voluntary and, according to former Environmental Protection Agency scientist Doug Gurian-Sherman, PhD, �often lack[s] sufficient detail, such as necessary statistical analyses needed for an adequate safety evaluation.� Using Freedom of Information Requests, Dr. Gurian-Sherman analyzed more than a fourth of the data summaries (14 of 53) of GM crops reviewed by the FDA. He says, �The FDA consultation process does not allow the agency to require submission of data, misses obvious errors in company- submitted data summaries, provides insufficient testing guidance, and does not require sufficiently detailed data to enable the FDA to assure that GE crops are safe to eat.�94 Similarly, a Friends of the Earth review of company and FDA documents concluded:
Submissions to the US Food and Drug Administraion (FDA) may be worse than in other countries, since the agency doesn�t actually require any data. Their policy says that biotech companies can determine if their own foods are safe. Anything submitted is voluntary and, according to former Environmental Protection Agency scientist Doug Gurian-Sherman, PhD, �often lack[s] sufficient detail, such as necessary statistical analyses needed for an adequate safety evaluation.� Using Freedom of Information Requests, Dr. Gurian-Sherman analyzed more than a fourth of the data summaries (14 of 53) of GM crops reviewed by the FDA. He says, �The FDA consultation process does not allow the agency to require submission of data, misses obvious errors in company- submitted data summaries, provides insufficient testing guidance, and does not require sufficiently detailed data to enable the FDA to assure that GE crops are safe to eat.�94 Similarly, a Friends of the Earth review of company and FDA documents concluded: The unpublished industry studies submitted to regulators are typically kept secret based on the claim that it is �confidential business information.� The Royal Society of Canada is one of many organizations that condemn this practice. They wrote:
The unpublished industry studies submitted to regulators are typically kept secret based on the claim that it is �confidential business information.� The Royal Society of Canada is one of many organizations that condemn this practice. They wrote: In addition, to relying on untested assumptions, industry-funded research is often designed specifically to force a conclusion of safety. In the high lysine corn described above, for example, the levels of certain nutritional components (i.e. protein content, total dietary fiber, acid detergent fiber, and neutral detergent fiber) were far outside the normal range for corn. Instead of comparing their corn to normal controls, which would reveal this disparity, Monsanto compared it to obscure corn varieties that were also substantially outside the normal range on precisely these values. Thus, their study found no statistical differences by design.
In addition, to relying on untested assumptions, industry-funded research is often designed specifically to force a conclusion of safety. In the high lysine corn described above, for example, the levels of certain nutritional components (i.e. protein content, total dietary fiber, acid detergent fiber, and neutral detergent fiber) were far outside the normal range for corn. Instead of comparing their corn to normal controls, which would reveal this disparity, Monsanto compared it to obscure corn varieties that were also substantially outside the normal range on precisely these values. Thus, their study found no statistical differences by design. Monsanto�s 1996 Journal of Nutrition studies on Roundup Ready soybeans107,108 provide plenty of examples of scientific transgressions. Although the study has been used often by the industry as validation for safety claims, experts working in the field were not impressed. For example, Dr. Arpad Pusztai was commissioned at the time by the UK government to lead a 20 member consortium in three institutions to develop rigorous testing protocols on GM foods�protocols that were never implemented. Dr. Pusztai, who had published several studies in that same nutrition journal, said the Monsanto paper was not �up to the normal journal standards.� He said, �It was obvious that the study had been designed to avoid finding any problems. Everybody in our consortium knew this.� Some of the flaws include:
Monsanto�s 1996 Journal of Nutrition studies on Roundup Ready soybeans107,108 provide plenty of examples of scientific transgressions. Although the study has been used often by the industry as validation for safety claims, experts working in the field were not impressed. For example, Dr. Arpad Pusztai was commissioned at the time by the UK government to lead a 20 member consortium in three institutions to develop rigorous testing protocols on GM foods�protocols that were never implemented. Dr. Pusztai, who had published several studies in that same nutrition journal, said the Monsanto paper was not �up to the normal journal standards.� He said, �It was obvious that the study had been designed to avoid finding any problems. Everybody in our consortium knew this.� Some of the flaws include: When governments fail in their duty to keep corporations in check, the �protector� role should shift to the media, which acts as a watchdog to expose public dangers and governmental shortcomings. But mainstream media around the world has largely overlooked the serious problems associated with GM crops and their regulation. The reason for this oversight is varied and includes contributions from an aggressive public relations and disinformation campaign by the biotech industry, legal threats by biotech companies, and in some cases, the fear of losing advertising accounts. This last reason is particularly prevalent among the farm press, which receives much of its income from the biotech industry.
When governments fail in their duty to keep corporations in check, the �protector� role should shift to the media, which acts as a watchdog to expose public dangers and governmental shortcomings. But mainstream media around the world has largely overlooked the serious problems associated with GM crops and their regulation. The reason for this oversight is varied and includes contributions from an aggressive public relations and disinformation campaign by the biotech industry, legal threats by biotech companies, and in some cases, the fear of losing advertising accounts. This last reason is particularly prevalent among the farm press, which receives much of its income from the biotech industry. One of the most troubling aspects of the biotech debate is the attack strategy used on GMO critics and independent scientists. Not only are adverse findings by independent scientists often suppressed, ignored, or denied, researchers that discover problems from GM foods have been fired, stripped of responsibilities, deprived of tenure, and even threatened. Consider Dr. Pusztai, the world�s leading scientist in his field, who inadvertently discovered in 1998 that unpredictable changes in GM crops caused massive damage in rats. He went public with his concerns, was a hero at his prestigious institute for two days, and then, after the director received two phone calls allegedly from the UK Prime Minister�s office, was fired after 35 years and silenced with threats of a lawsuit. False statements were circulated to trash his reputation, which are recited by GMO advocates today.
One of the most troubling aspects of the biotech debate is the attack strategy used on GMO critics and independent scientists. Not only are adverse findings by independent scientists often suppressed, ignored, or denied, researchers that discover problems from GM foods have been fired, stripped of responsibilities, deprived of tenure, and even threatened. Consider Dr. Pusztai, the world�s leading scientist in his field, who inadvertently discovered in 1998 that unpredictable changes in GM crops caused massive damage in rats. He went public with his concerns, was a hero at his prestigious institute for two days, and then, after the director received two phone calls allegedly from the UK Prime Minister�s office, was fired after 35 years and silenced with threats of a lawsuit. False statements were circulated to trash his reputation, which are recited by GMO advocates today. Since GM foods are not properly tested before they enter the market, consumers are the guinea pigs. But this doesn�t even qualify as an experiment. There are no controls and no monitoring. Given the mounting of evidence of harm, it is likely that GM foods are contributing to the deterioration of health in the United States, Canada, and other countries where it is consumed. But without post- marketing surveillance, the chances of tracing health problems to GM food are low. The incidence of a disease would have to increase dramatically before it was noticed, meaning that millions may have to get sick before a change is investigated. Tracking the impact of GM foods is even more difficult in North America, where the foods are not labeled.
Since GM foods are not properly tested before they enter the market, consumers are the guinea pigs. But this doesn�t even qualify as an experiment. There are no controls and no monitoring. Given the mounting of evidence of harm, it is likely that GM foods are contributing to the deterioration of health in the United States, Canada, and other countries where it is consumed. But without post- marketing surveillance, the chances of tracing health problems to GM food are low. The incidence of a disease would have to increase dramatically before it was noticed, meaning that millions may have to get sick before a change is investigated. Tracking the impact of GM foods is even more difficult in North America, where the foods are not labeled.






