
by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gastro Intestinal Health, Gut and Intestinal Health, Health, Wellness
Do you feel:
- Edema and swelling in the ankles and the wrist?
- Muscle cramping?
- Frequent urination?
- Poor muscle endurance?
- Alternation in bowel regularity?
If you are experiencing any of these situations, then you might be experiencing chronic kidney disease.
About over 10% of the adult population suffers from CKD (chronic kidney disease), and the two leading underlying causes of the end-stage of chronic kidney disease are type 2 diabetes and hypertension. Other chronic ailments like dysbiosis of the gut microbiome, inflammation, oxidative stress, as well as environmental toxins and PPI (proton pump inhibitor). All these chronic ailments have been linked to chronic kidney disease in the body.
Chronic Kidney Disease
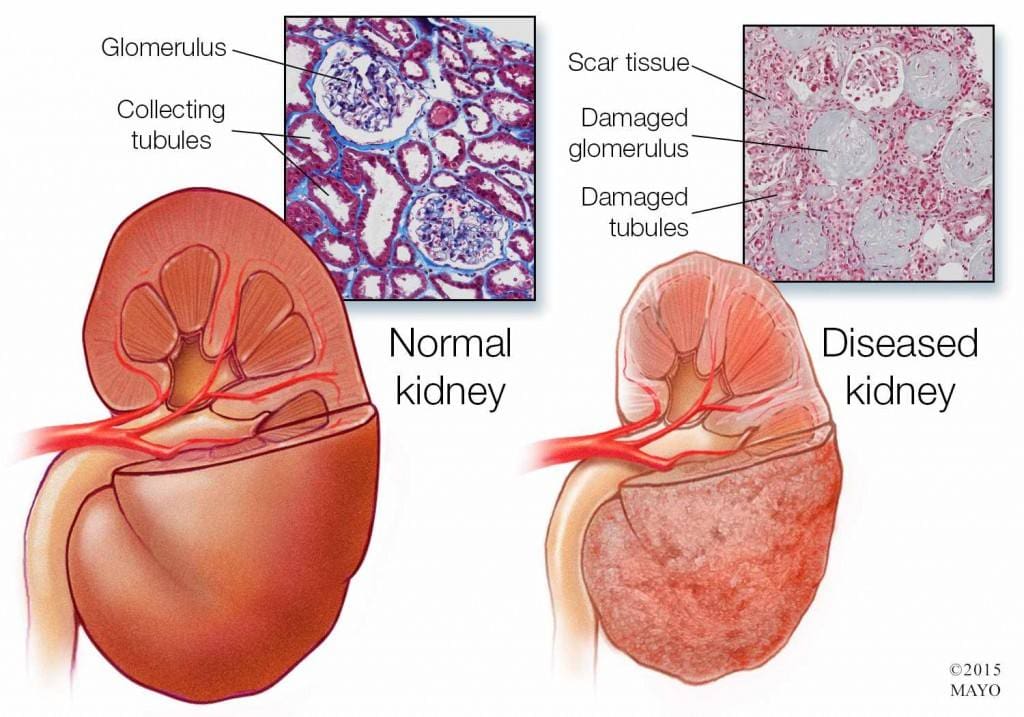
Chronic kidney disease is a slow and progressive loss of kidney function over several years. Also known as chronic renal failure, it much more widespread, and it often goes undetected and undiagnosed until the disease is well advanced. It is not unusual for anyone to realize they have chronic kidney failure when their kidneys are functioning only at 25% than average. As it advances and the kidney’s function is severely impaired, dangerous levels of waste and fluid can rapidly build up in the body.
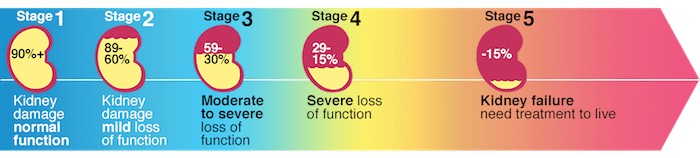
Chronic kidney failure is different from acute kidney failure due to being a slow and gradually progressive disease. When the disease is fairly well advanced, the conditions are more severe than the signs and symptoms are noticeable, making most of the damage irreversible. Here are some of the most common signs and symptoms of chronic kidney disease include:
- Anemia
- Blood in urine
- Dark urine
- Edema- swollen feet, hands, ankles, and face
- Fatigue
- Hypertension
- More frequent urination, especially at night
- Muscle cramps and twitches
- Pain on the side or mid to lower back
Dietary Fibers for CKD
Researchers have investigated that the role of dietary fibers and the gut microbiome is in renal diets. When there is a dysbiosis in the gut microbiome, it can be a risk factor for the development of chronic kidney disease, thus reducing the renal function. That function will significantly contribute to dysbiosis. The current renal dietary recommendations include a reduction of protein intake with an increase in complex carbohydrates and fiber.
A systemic review did a test that included 14 controlled trials and 143 participants that had chronic kidney disease. The test demonstrated that all 143 participants had a reduction in serum creatinine and urea that is associated with dietary fiber intake, which occurs in a dose-dependent matter. These participants had an average intake of 27 grams of fiber per day in their diet. It is also an essential note that creatinine is metabolized in the intestinal bacteria in the body.

A high fiber diet can lead to the production of SCFAs (short-chain fatty acids) in the gastrointestinal tract. They play an essential role in T regulatory cell activation, which regulates the intestinal immune system. When there is dysregulation in the immune system, it can cause an increase of inflammation that may occur in chronic kidney disease. With a high fiber diet, the intake is associated with lowering the risk of inflammation and the mortality in kidney disease.
Increasing fiber intake is relatively easy with some of these high fiber foods that are both healthy and nutritious and can help individual’s that have kidney disease. These include:
- Pears
- Strawberries
- Avocados
- Apples
- Carrots
- Beets
- Broccoli
- Lentils
Research has previously demonstrated that a high fiber diet for CDK patients is characterized by the control increase of plant-origin protein and animal-origin foods. This is useful for individuals to limit the consumption of processed food products because of modern conservation processes, which has the purpose of eliminating pathogenic bacteria. People who have chronic kidney disease that go on a high fiber diet have been linked to better kidney function and lowering the risk of inflammation and mortality.
Some individuals may experience some gastrointestinal side effects when they are trying to increase their fiber intake.�Research has been stated that patients should consider resistant starches since it has shown no side effects with the recommended doses.
Conclusion
Chronic kidney disease is a slow and progressive loss of kidney function. The signs and symptoms are noticeable as the disease progress in the later stages. With a high fiber diet, individuals can lower the risk of inflammation and mortality of CDK. When this disease causes inflammation and chronic illness in the kidneys, complications can travel through the entire body. The high fiber diet can also be beneficial for the gut microbiome to function correctly, and some products can help lower the stress hormones and make sure that the body’s hypothalamic-pituitary-adrenal axis is functioning correctly.
October is Chiropractic Health Month. To learn more about it, check out Governor Abbott�s bill on our website to get full details on this historic moment.
The scope of our information is limited to chiropractic, musculoskeletal and nervous health issues as well as functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or chronic disorders of the musculoskeletal system. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900 .
References:
D�Alessandro, Claudia. �Dietary Fiber and Gut Microbiota in Renal Diets.� MDPI, Multidisciplinary Digital Publishing Institute, 9 Sept. 2019, www.mdpi.com/2072-6643/11/9/2149/htm.
Gunnars, Kris. �22 High-Fiber Foods You Should Eat.� Healthline, 10 Aug. 2018, www.healthline.com/nutrition/22-high-fiber-foods.
Jurgelewicz, Michael. �New Article Investigates the Role of Dietary Fiber and the Gut Microbiome in Chronic Kidney Disease.� Designs for Health, 13 Sept. 2019, blog.designsforhealth.com/node/1105.
Khosroshahi, H T, et al. �Effects of Fermentable High Fiber Diet Supplementation on Gut Derived and Conventional Nitrogenous Product in Patients on Maintenance Hemodialysis: a Randomized Controlled Trial.� Nutrition & Metabolism., U.S. National Library of Medicine, 12 Mar. 2019, www.ncbi.nlm.nih.gov/pubmed/?term=30911321.
Krishnamurthy, Vidya M Raj, et al. �High Dietary Fiber Intake Is Associated with Decreased Inflammation and All-Cause Mortality in Patients with Chronic Kidney Disease.� Kidney International, U.S. National Library of Medicine, Feb. 2012, www.ncbi.nlm.nih.gov/pmc/articles/PMC4704855/.
Newman, Tim. �Chronic Kidney Disease: Symptoms, Causes, and Treatment.� Medical News Today, MediLexicon International, 13 Dec. 2017, www.medicalnewstoday.com/articles/172179.php.
Staff, Mayo Clinic. �Chronic Kidney Disease.� Mayo Clinic, Mayo Foundation for Medical Education and Research, 15 Aug. 2019, www.mayoclinic.org/diseases-conditions/chronic-kidney-disease/symptoms-causes/syc-20354521.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gastro Intestinal Health, Gut and Intestinal Health, Health, Nutrition, Wellness
Do you feel:
- Inflammation in your joints?
- Unpredictable abdominal swelling?
- Frequent bloating and distention after eating?
- Unpredictable food reactions?
- Aches, pains, and swelling throughout the body?
If you are experiencing any of these situations, then you might be experiencing a low intake of fiber in your diet, causing inflammation.
Throughout several decades, Americans have lost much diversity in their diets, impacting their gut microbiome, and the contribution to the autoimmune disorder epidemic. The vast majority of people have a less than perfect diet that is consists of high in calories, short on nutrients, and low on fiber intake. Research has stated that about only 10 percent of Americans have met their daily fiber requirements.
The diet is a significant environmental trigger in autoimmune diseases. Dietary approaches can provide the most effective means of an individual to returning balance and the dysfunction with the gastrointestinal system. Researchers have found out that the role of dietary fibers can help with rheumatoid arthritis as there is new and developing research on this discovery.
What is Rheumatoid Arthritis?
Rheumatoid arthritis is a long term, progressive, and disabling autoimmune disease. It causes inflammation, swelling, and pain in and around the joints and organs of the body. It affects up to 1 percent of the world’s population and over 1.3 million people in America, according to the Rheumatoid Arthritis Support Network.

Rheumatoid arthritis is also a systemic disease, which means that it affects the whole body, not just the joints. It occurs when an individual’s immune system mistakes their body’s healthy tissues for foreign invaders. As the immune system responds to this, inflammation occurs in the target tissue or organ. Symptoms of rheumatoid arthritis can include:
- Pain, swelling, and stiffness in more than one joint
- Symmetrical joint involvement
- Joint deformity
- Unsteadiness when walking
- Fever
- A general feeling of being unwell
- Loss of function and mobility
- Weight loss
- Weakness
Fiber and Inflammation
Individuals who eat healthily knows that eating fibers in their diet can help reduce the risk of developing various conditions. The AHAEP (American Heart Association Eating Plan) has stated that people should be eating a variety of food fiber sources in their diet. The total dietary fiber intake that a person should be eating is 25 to 30 grams a day from foods, not supplements. Currently, adults in the United States eat about 15 grams a day on their fiber, which is half of the recommended amount.
Eating a high fiber diet can provide many rewards to the body. Eating fruits, vegetables, beans, nuts, and whole grains can provide a boost of vitamins, minerals, protein, and healthy nutrients in the body. Studies have been shown that eating a high fiber diet can help lower the markers of inflammation, which is a critical factor in many forms of arthritis.

The body needs two types of fibers, which are soluble and insoluble. Soluble fibers are mixed with water to form a gel-like consistency, which slows digestion and helps the body absorb nutrients better and helps lower total cholesterol and LDL cholesterol. Insoluble fibers help the digestive system run more efficiently as it adds bulk to stool, which can help prevent constipation.
There have been a few studies that found that people who eat high fiber diets have lower CRP (C-reactive protein) levels in their blood. CRP is a marker for inflammation and is linked to rheumatoid arthritis. When a person eats a high fiber diet, it not only reduces inflammation to their bodies, but it helps lower the body weight as well. High fiber-rich foods feed the beneficial bacteria living in the gut, and then it is releasing substances to the body, promoting lower levels of inflammation.
A study has been shown that patients with rheumatoid arthritis that they consumed either a high fiber bar or cereal for 28 days while continuing with their current medication had decreased levels of inflammation. Researchers noticed that they had an increase of T regulatory cell numbers, a positive Th1/Th17 ratio, a decrease in bone erosion, and a healthy gut microbiome.
Gut Health and Inflammation

The gut plays a crucial role in the immune function as well as digesting and absorbing food in the body. The intestinal barrier provides an effective protective barrier from pathogenic bacteria but also being a healthy environment for beneficial bacteria. With a high fiber diet, it can lead to the production of SCFAs (short-chain fatty acids) in the gastrointestinal tract, thus playing an essential role in T regulatory cell activation, which regulates the intestinal immune system. When inflammation comes to play in the gut, it can disrupt the intestinal permeability barrier and cause a disruption, leading to leaky gut. Probiotics and a high fiber diet can help prevent inflammation and provide a healthy gut function.
Conclusion
Eating a high fiber diet is essential to prevent inflammation, not on the joints, but everywhere in the body. Even though individuals eat half of the recommended amount of fiber in their diets, due to their hectic lifestyle, eating a high fiber diet is beneficial. Incorporating fiber in their diet gradually is ideal as well as drinking water with the fibers to make the process work more effectively in the body. Some products can help aid the body by supporting not only the gastrointestinal function and muscular system but making sure that the skin, hair, nail, and joints are healthy as well.
October is Chiropractic Health Month. To learn more about it, check out Governor Abbott�s declaration on our website to get full details on this historic moment.
The scope of our information is limited to chiropractic, musculoskeletal and nervous health issues as well as functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or chronic disorders of the musculoskeletal system. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900 .
References:
at UCSF Medical Center, Healthcare Specialist. �Increasing Fiber Intake.� UCSF Medical Center, 2018, www.ucsfhealth.org/education/increasing_fiber_intake/.
Brazier, Yvette. �Rheumatoid Arthritis (RA): Symptoms, Causes, and Complications.� Medical News Today, MediLexicon International, 16 Oct. 2018, www.medicalnewstoday.com/articles/323361.php.
Hakansson, Asa, and Goran Molin. �Gut Microbiota and Inflammation.� Nutrients, MDPI, June 2011, www.ncbi.nlm.nih.gov/pmc/articles/PMC3257638/.
Jurgelewicz, Michael. �New Study Demonstrates the Role of Fiber in Rheumatoid Arthritis.� Designs for Health, 11 Oct. 2019, blog.designsforhealth.com/node/1125.
Unknown, Unknown. �More Fiber, Less Inflammation?� Www.arthritis.org, 25 June, 2015, www.arthritis.org/living-with-arthritis/arthritis-diet/anti-inflammatory/fiber-inflammation.php.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gastro Intestinal Health, Gut and Intestinal Health, Health, Nutrition, Wellness
Do you feel:
- Feel hungry an hour or two after eating
- Digestive problems subside with rest
- Excessive belching, burping or bloating
- Stomach pain, burning or aching 1-4 hours after eating
- Sense of fullness during and after meals
If you are experiencing any of the situations, then you might want to try these six types of food to help boost your immune system.
The Immune System
The immune system is the body�s defense mechanism that provides a robust anatomical barrier.� The gastrointestinal tract is one of the barriers. It has many defense mechanisms such as peristalsis, gastric acid, bile acids, digestive enzymes, flushing, thiocyanate, defensins, and gut flora in the body. The gut flora is the critical focus for many health professionals; however, all the essential defense mechanisms rely heavily on the gastrointestinal tract to function efficiently.
There are ways to benefit the immune system as one of the ways is to plan meals that are filled with necessary nutrients that can fight off infections. Prebiotic and probiotic-rich foods help enhance microbial diversity in the gut, while vitamin C-rich foods can mop up the free radicals that have entered the body. Another benefit is to avoid foods that promote infections like heavily processed foods, added sugars, and sodas. When it is not consumed in the body, it can help boost immunity and enrich the gut microbiome. Here are the six foods to help boost the immune system in the body.
Yellow Bell Peppers

Due to being the most natural vegetable to find at a local grocery store or farmer’s markets around the world, yellow bell peppers contain more vitamin C than oranges. Since oranges contain about 78% of vitamin C, yellow bell peppers contain about 152% of vitamin C and numerous vitamins and minerals. Bell peppers (yellow, red, orange and green) contain the following:
- Vitamin B6: Bell peppers contain pyridoxine, which is an essential nutrient for the formation of red blood cells.
- Vitamin K1: This vitamin is also known as phylloquinone, which is vital for bone health and blood clotting.
- Potassium: This mineral is essential for improving heart health.
- Folate: Also known as vitamin B9, this vitamin has a variety of functions to the body and is highly essential to take during pregnancy.
- Vitamin E: This is a powerful antioxidant that is essential for healthy nerves and muscles.
- Vitamin A: Red bell peppers are high in beta carotene when consumed converts to vitamin A in the body.
Vitamin C helps boost the immune system by influencing the development and function of lymphocytes, and with about half a cup of yellow bell peppers will give the body those lymphocytes.
Guava
Guava is a traditional remedy for a range of health conditions that a person may encounter. These tropical fruits are seasonal throughout the winter. They contain about 140% of vitamin C and rich with lycopene, which is excellent for the immune system as it plays an essential role in the activities of the enzymes. Lycopene is a powerful antioxidant that has been implicated in having a potentially beneficial impact on several chronic diseases, including cancer.

Studies have been shown that the guava fruit and the leaves have been known to have a positive effect on a range of illnesses and symptoms, including:
- Type 2 diabetes
- Menstrual cramps
- Diarrhea
- Flu
- Blood pressure
- Osteoarthritis
- Cancer
Broccoli

Broccoli is high in phytonutrients like vitamins A, C, and E while also containing sulforaphane. Sulforaphane is activated when broccoli or any cruciferous vegetables are chewed, cut, or damaged. Raw broccoli or broccoli sprouts contain the highest level of sulforaphane when it is not boiled or cooked. Studies have been shown that consuming broccoli has been associated with reducing many lifestyle-related health conditions like:
- Obesity
- Diabetes
- Improves digestion
- Regulate the immune system
- Helps support healthy-looking skin
- Decrease inflammation
- Lowers blood pressure
Turmeric
Turmeric is an excellent immune-boosting food since it supports healthy inflammatory pathways in the body. Inflammation in the body is implicated in the pathophysiology of many health-compromising situations that can lead to chronic illnesses. So consuming pro healthy inflammation foods like turmeric or incorporating turmeric in dishes is an ideal way to boost the immune system.

The active component in turmeric is curcumin and has potent biological properties like anti-oxidative, anti-cytotoxic, and neurorestorative properties, making it an essential staple in an immune-boosting food. Here are some of the benefits that turmeric provides to the body:
- Anti-inflammatory properties
- Pain relief on the joints
- Improves liver function
- Reducing the risk of cancer
- Preventing gut inflammation
Green Tea

Green tea helps the body relax and contains L-theanine that helps the formation of healthy T-cells. Green tea also contains EGCG (epigallocatechin gallate) and is packed filled with flavonoids to help boost the body’s immune system. Here are some of the health benefits that green tea provides:
- Cancer prevention
- Lowers the risk of cardiovascular diseases
- Lowers cholesterol
- Decrease the risk of a stroke
- Lowers the risk of type 2 diabetes
- Help lose weight
- Helps lowers inflammation on the skin
- Improves brain function
- Helps reduce the risk of Alzheimer�s disease
Almonds

Almonds are packed filled with vitamins, minerals, protein, and fibers. It contains vitamin E and helps boost the immune system since it is a free radical scavenging antioxidant. They are easy to find in any grocery store, and the health benefits that almonds can provide are:
- Lowering cholesterol
- Reduce the risk of cancer
- Provide heart health benefits
- Reduce type 2 diabetes
- Manage weight
Conclusion
Eating these six foods can be beneficial to support a healthy immune system. They are bursting with plant-based nutrition that the body needs to make sure that chronic illnesses like inflammation in the gut. Some products help support the immune system as well as making sure that the gastrointestinal system and the sugar metabolism is supported. Eating a variety of foods that has antioxidants and anti-inflammatory properties is beneficial to the body. With the cold and flu season approaching, it is highly relevant to consume these foods to help fight against the cold and flu and providing assistance to the immune system.
October is Chiropractic Health Month. To learn more about it, check out Governor Abbott�s declaration on our website to get full details on this historic moment.
The scope of our information is limited to chiropractic, musculoskeletal and nervous health issues as well as functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or chronic disorders of the musculoskeletal system. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900 .
Reference:
Ahmed, Touqeer, et al. �Curcuminoids Rescue Long-Term Potentiation Impaired by Amyloid Peptide in Rat Hippocampal Slices. – Semantic Scholar.� Undefined, 1 Jan. 1970, www.semanticscholar.org/paper/Curcuminoids-rescue-long-term-potentiation-impaired-Ahmed-Gilani/c66297f8d0f3b633fac263cbb81f82de1893387a.
Arnarson, Atli. �Bell Peppers 101: Nutrition Facts and Health Benefits.� Healthline, 27 Mar. 2019, www.healthline.com/nutrition/foods/bell-peppers.
Burgess, Lana. �Health Benefits of Guava: How to Use It, Nutrition, and Risks.� Medical News Today, MediLexicon International, 20 Mar. 2019, www.medicalnewstoday.com/articles/324758.php.
Du, Guang-Jian, et al. �Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea.� Nutrients, MDPI, 8 Nov. 2012, www.ncbi.nlm.nih.gov/pmc/articles/PMC3509513/.
Kim, DS, et al. “Curcuminoids from Curcuma Longa L. (Zingiberaceae) That Protect PC12 Rat Pheochromocytoma and Normal Human Umbilical Vein Endothelial Cells from BetaA(1-42) Insult.� Neuroscience Letters, U.S. National Library of Medicine, 27 Apr. 2001, www.ncbi.nlm.nih.gov/pubmed/11297823.
Luo, Cong, and Xian-Guo Wu. �Lycopene Enhances Antioxidant Enzyme Activities and Immunity Function in N-Methyl-N’-Nitro-N-Nitrosoguanidine-Enduced Gastric Cancer Rats.� International Journal of Molecular Sciences, Molecular Diversity Preservation International (MDPI), 2011, www.ncbi.nlm.nih.gov/pmc/articles/PMC3116194/.
Menon, Venugopal P, and Adluri Ram Sudheer. �Antioxidant and Anti-Inflammatory Properties of Curcumin.� Advances in Experimental Medicine and Biology, U.S. National Library of Medicine, 2007, www.ncbi.nlm.nih.gov/pubmed/17569207.
Nordqvist, Joseph. �Almonds: Health Benefits, Nutrition, and Risks.� Medical News Today, MediLexicon International, 14 Dec. 2017, www.medicalnewstoday.com/articles/269468.php.
Team, Biotics Education. �Key Foods to Boost the Immune System.� Biotics Research Blog, 15 Oct. 2019, blog.bioticsresearch.com/key-foods-to-boost-the-immune-system.
van Gorkom, Gwendolyn N Y, et al. �Influence of Vitamin C on Lymphocytes: An Overview.� Antioxidants (Basel, Switzerland), MDPI, 10 Mar. 2018, www.ncbi.nlm.nih.gov/pubmed/29534432.
Vermeulen, Martijn, et al. �Bioavailability and Kinetics of Sulforaphane in Humans after Consumption of Cooked versus Raw Broccoli.� Journal of Agricultural and Food Chemistry, U.S. National Library of Medicine, 26 Nov. 2008, www.ncbi.nlm.nih.gov/pubmed/18950181.
Ware, Megan. �Broccoli: Health Benefits, Nutritional Information.� Medical News Today, MediLexicon International, 8 Dec. 2017, www.medicalnewstoday.com/articles/266765.php.
Ware, Megan. �Green Tea: Health Benefits, Side Effects, and Research.� Medical News Today, MediLexicon International, 28 Mar. 2017, www.medicalnewstoday.com/articles/269538.php.
Ware, Megan. �Turmeric: Benefits and Nutrition.� Medical News Today, MediLexicon International, 24 May 2018, www.medicalnewstoday.com/articles/306981.php.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gastro Intestinal Health, Gut and Intestinal Health, Health, Nutrition, Vitamins, Wellness
Do you feel:
- Excessive belching, burping or bloating
- Gas immediately following a meal
- Stomach pain, burning or aching 1-4 hours after eating
- Feel hungry an hour or two after eating
- Digestive problems when lying down or bending forward
If you are experiencing any of these situations, then you should try some micronutrients for your GI tract health.
GI Tract Health

Over two thousand years ago, Hippocrates recognized that the gut plays a significant role in overall health and that modern scientific research has substantiated and solidified this view. With GI (gastrointestinal) health, advanced testing, and intricate healing protocols focused a lot when it comes to the GI tract. Some patients may benefit from the precise analysis of the makeup from their gut flora or the specific food elimination and reintroduction strategies, but not to overlook the fundamentals. Addressing the basics like general micronutrient repletion or supplementation with the foundational nutrients can be targeted for therapeutic purposes and can go a long way for an individual�s healing.
The Micronutrients
These are some of the fundamental micronutrients that the body needs to perform the everyday task. These can mostly be found in foods or in supplements and vitamins that are consumed, and even though high restriction diets can deplete these nutrients, they are still crucial for not only our gut health but for the entire body system as well.
Glutamine
The amino acid glutamine is a trusty workhorse for a healthy gut function in the body. Even though it is technically not an essential amino acid, it serves as an energy source for epithelial cells that makes up the intestinal lining for the intestines. Various circumstances like trauma, burns, or recovery from significant operations or illnesses can increase the body’s demand for glutamine.
Glutamine can be found in all protein foods like:
- Eggs
- Beef
- Skim milk
- Tofu
- White rice
- Corn
Taurine
Another amino acid is taurine is beneficial for individuals who need help with the digestion of dietary fats. Taurine is unique due to not being used in any structural protein; however, it has other roles in the body. Taurine can be synthesized from cysteine and can be obtained from animal foods specifically, sadly though it is nonexistent in plant food. Bile acids that are bound with taurine are secreted by the liver; the making of this compound is critical for bile acid function and proper fat absorption in the body.
Taurine can provide these health benefits to the body, which includes:
- Improve blood sugar control and fight diabetes
- Stop the occurrence of epilepsy
- Reduces seizure attacks
- Prevents cardiovascular diseases
- Regulates muscle contractions
- Controls and calms the central nervous system
Potassium
Potassium is the core nutrient that plays a role in a healthy GI function, especially when it comes to intestinal motility. Some disorders like fatigue and cardiac arrhythmias can be the result of potassium deficiency, and inadequate potassium may lead to delayed gastric emptying and intestinal paralysis. If the body is not treated soon, it can lead to chronic illnesses in the GI, causing unpleasant effects like bloating, abdominal pain, and constipation.
All food supplies have an abundance of potassium, but certain medications can reduce potassium levels. Factors like excessive alcohol consumption or strict chronic dieting for weight loss can be the result of inadequate potassium intake and the body status of a person.
Some of the health benefits that potassium can provide are:
- Maintains constant blood pressure
- Reduce the risk of cardiovascular diseases
- Maintains bone density
- Maintains muscle mass
Vitamin B6
B vitamins, especially vitamin B6, are highly essential to the GI tract because they make sure that the brain is also healthy as well. Deficiency of vitamin B6 can cause these symptoms:
- Tingling, numbness, and pain in the hands and feet
- Anemia
- Seizures
- Depression
- Confusion
- Weak immune system
Vitamin B6 is a water-soluble vitamin that produces the neurotransmitters serotonin and norepinephrine and forming myelin for the body. This vitamin can help boost brain function and can improve memory function. Some of the other benefits it can provide to the body are:
- Lowers the risk of dementia
- Reduce the severity of nausea during pregnancy
- Protection from air pollution
- Ensures the normal functioning of digestive enzymes
Conclusion
Even though these are the necessary foundational micronutrients and amino acids for their roles in the GI tract, it is crucial for individuals who have these micronutrient deficiencies. Even though the popularity of highly restrictive diets emphasizes on caloric restrictions for weight loss for individuals, it can limit the intake of certain nutrient-dense foods. It can cause disruptions to the gastrointestinal tract. When a person surrounds themselves with an abundance of foods with these micronutrients can live a healthy life. Some products combined with these micronutrient foods can provide support to the gastrointestinal system and help boost the sugar metabolism for the body.
October is Chiropractic Health Month. To learn more about it, check out Governor Abbott�s declaration on our website to get full details on this historic moment.
The scope of our information is limited to chiropractic, musculoskeletal and nervous health issues as well as functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or chronic disorders of the musculoskeletal system. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900 .
References:
Brazier, Yvette. �Vitamin B-6: Benefits, Dosage, Food Sources, and Deficiency Symptoms.� Medical News Today, MediLexicon International, 27 Mar. 2017, www.medicalnewstoday.com/articles/219662.php.
Cadman, Bethany. �L-Glutamine for IBS: Benefits, Side Effects, and Research.� Medical News Today, MediLexicon International, 7 Feb. 2018, www.medicalnewstoday.com/articles/320850.php.
Caporuscio, Jessica. �What Is Taurine? Benefits and Side Effects.� Medical News Today, MediLexicon International, 26 Sept. 2019, www.medicalnewstoday.com/articles/326476.php.
Higdon, Jane. �Potassium.� Linus Pauling Institute, 14 Oct. 2019, lpi.oregonstate.edu/mic/minerals/potassium#deficiency.
Mawer, Rudy. “What Is Taurine? Benefits, Side Effects, and More.” Healthline, 27 Nov. 2018, www.healthline.com/nutrition/what-is-taurine.
Megan Ware, RDN. �Potassium: Health Benefits and Recommended Intake.� Medical News Today, MediLexicon International, 10 Jan. 2018, www.medicalnewstoday.com/articles/287212.php.
Team, DFH. �Micronutrients in GI Health.� Designs for Health, 11 Oct. 2019, blog.designsforhealth.com/node/1123.
Tinsley, Grant. “Glutamine: Benefits, Uses, and Side Effects.” Healthline, 13 Jan. 2018, www.healthline.com/nutrition/glutamine.
by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gastro Intestinal Health, Gut and Intestinal Health, Health, Wellness
Do you feel:
- Stomach pain
- Burning or aching after 1-4 hours of eating
- Use antacids frequently
- Heartburn
- Digestive problems subside with relaxation
If you are experiencing any of these situations, then you might be experiencing problems with your stomach acid pH balance.
The pH Balance of the Stomach
The stomach produces gastric acids that help breakdown the food contents that a person eats. With the gastric acids, studies stated that its role is diverting the bile and pancreatic juice from the intestines. With humans, the stomach plays a significant role as a biological filter with moderate lifestyle changes. Whether it changes in a person’s diet, hygiene, and medical interventions can alter the stomach’s pH levels.
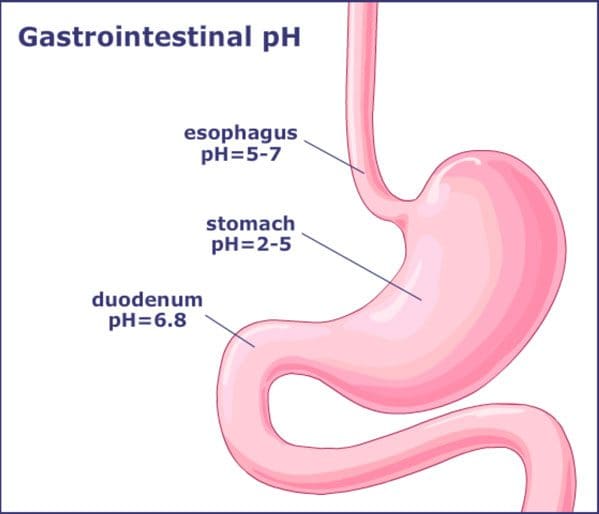
With the stomach acidity in the body, it is a double-edged sword. High acidity in the stomach can prevent pathogen exposure, but it can also decrease the likelihood of recolonization of beneficial microbes. Low acidity in the stomach is more likely to be colonized by pathogens and can cause gastric infections.
Acid Reflux
Acid reflux is a common condition that features a burning pain in the lower chest area, and it occurs when stomach acid flows back up into the food pipe. Diseases that are the result of acid reflux is one of the most common gut complaints from individuals and seen by hospital departments in the United States. The stomach contains hydrochloric acid that helps breakdown food and protects it from pathogens such as bacteria.
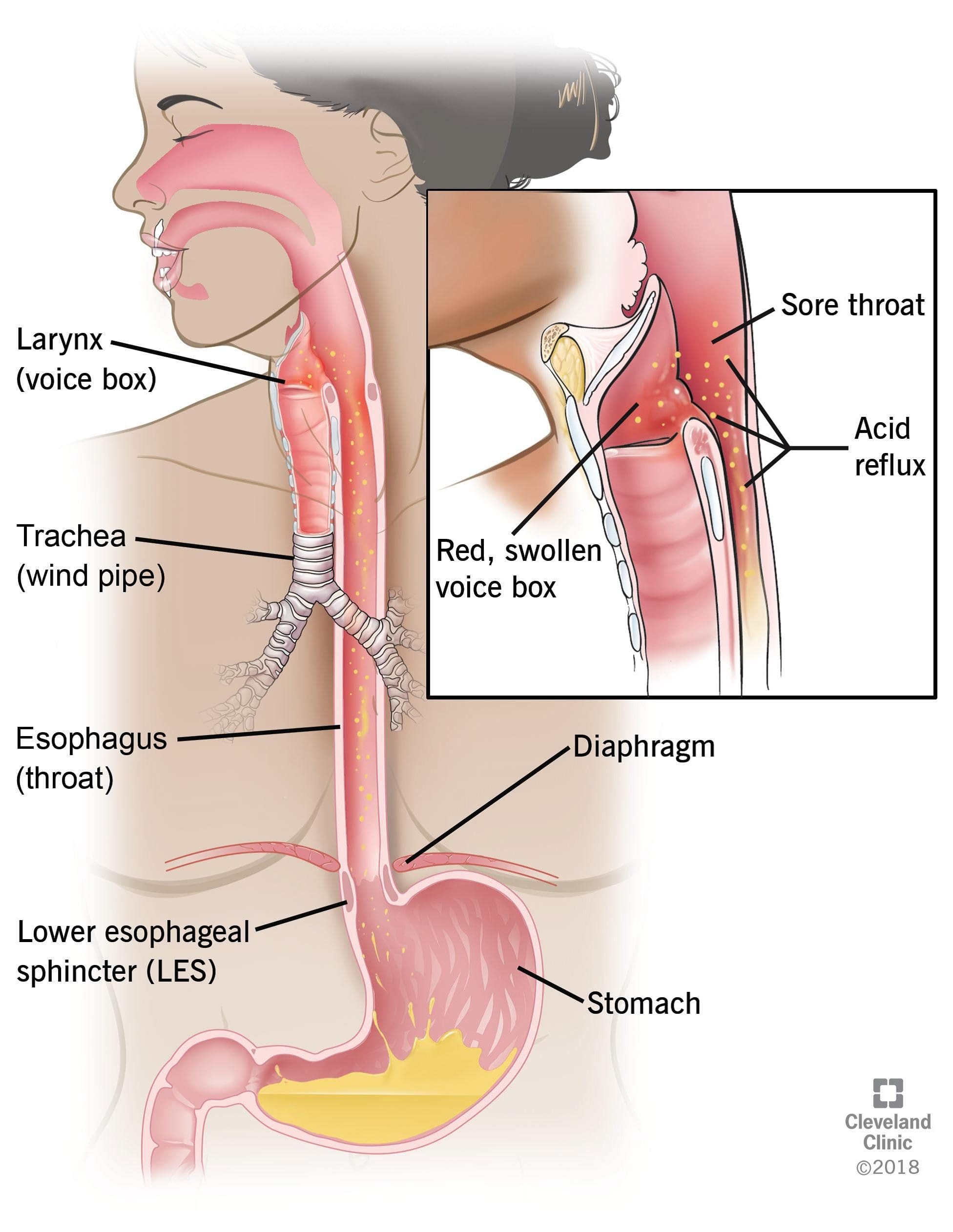
Even though the lining of the stomach is specially adapted to protect it from hydrochloric acid, the esophagus is not protected from this powerful acid. The gastroesophageal sphincter is a ring of muscle that generally acts as a valve that lets food into the stomach but does not let the food back up into the esophagus. When it fails, the stomach contents will regurgitate into the esophagus, and the symptoms of acid reflux will be felt.
One of the risk factors that acid reflux causes that are not preventable are hiatal hernia. This hernia causes a hole in the diaphragm that allows the upper part of the stomach to enter the chest cavity. Other risk factors include:
- Obesity
- Smoking (active or passive)
- Low levels of physical exercise
- Certain medication
- Poor diet
Some of the symptoms that acid reflux creates can cause heartburn, and it is uncomfortable when the sensation travels up to the neck and throat. When an individual lays down or bends over, it tends to get the worst and can last for several hours. Some of the symptoms caused by acid reflux include:
- Heartburn
- Sour taste in the mouth
- Regurgitation
- Dyspepsia
- Difficulty swallowing
- Sore throat
- Dry cough
- Asthma symptoms
Hypochlorhydria
Hypochlorhydria is the medical term for low levels of stomach acid. Individuals with hypochlorhydria are unable to produce enough hydrochloric acid in their stomach and may experience digestive issues, nutritional deficiencies, and gastrointestinal infections.
Some of the common causes of hypochlorhydria are:
- Age: Aging can make the stomach produce less acid in the body. A 2013 review stated that adults over the age of 65 are more susceptible to develop that hypochlorhydria.
- Stress: Even though everyday stress does not have much effect on the production of stomach acid, chronic stress, however, can contribute to hypochlorhydria.
- Medication: Individuals that use long-term antacids or other medication for acid reflux or heartburn may decrease the stomach acid that the body produces.
- Bacterial Infection: A bacteria called Helicobacter pylori is a widespread, yet under-appreciated pathogen that can alter the host physiology and subvert the host immune response. It is the primary cause of peptic ulcers and gastric cancers while contributing to a low level of stomach acid.
- Zinc deficiency: Zinc is a necessary mineral for stomach acid production. A lack of this mineral can contribute to hypochlorhydria to the body.
- Stomach surgery: Surgical procedures like gastric bypass surgery can reduce the amount of the stomach produces.
Symptoms of hypochlorhydria are related to impaired digestion, increase infection, and reduce the absorption of nutrients from food. Symptoms may include:
- Bloating
- Burping
- Upset stomach
- Heartburn
- Gas
- Indigestion
- Undigested food in stool
- Neurological issues like numbness, tingling, and vision changes
Conclusion
The stomach is producing gastric acids that help break down food components. When environmental factors are in effect, they can alter the stomach’s pH balance and can disrupt the hydrochloric acid. Since stomach acidity is a double edge sword, it can go back and forth on the pH levels. High acidity in the stomach can cause acid reflux to the esophagus and decrease the likelihood of recolonizing beneficial microbes in the gut. Low acidity in the stomach can cause hypochlorhydria and develop digestive issues, nutrient deficiencies, and gastrointestinal infections. These products can help support the gastrointestinal system, as well as supporting the pH-optimized enzymes in both the gastric and intestinal function in the body.
October is Chiropractic Health Month. To learn more about it, check out Governor Abbott�s proclamation on our website to get full details on this historic moment.
The scope of our information is limited to chiropractic, musculoskeletal and nervous health issues as well as functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or chronic disorders of the musculoskeletal system. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900 .
Reference:
Beasley, DeAnna E, et al. �The Evolution of Stomach Acidity and Its Relevance to the Human Microbiome.� PloS One, Public Library of Science, 29 July 2015, www.ncbi.nlm.nih.gov/pmc/articles/PMC4519257/.
Britton, Edward, and John T. McLaughlin. �Ageing and the Gut.� Cambridge Core, Cambridge University Press, 12 Nov. 2012, www.cambridge.org/core/journals/proceedings-of-the-nutrition-society/article/ageing-and-the-gut/A85D096755F5F7652C262495ABF302A0/core-reader.
Dix, Megan. �What Is Hypochlorhydria?� Healthline, 12 Mar. 2018, www.healthline.com/health/hypochlorhydria.
Green, G M. �Role of Gastric Juice in Feedback Regulation of Rat Pancreatic Secretion by Luminal Proteases.� Pancreas, U.S. National Library of Medicine, July 1990, www.ncbi.nlm.nih.gov/pubmed/2199966.
Kines, Kasia, and Tina Krupczak. �Nutritional Interventions for Gastroesophageal Reflux, Irritable Bowel Syndrome, and Hypochlorhydria: A Case Report.� Integrative Medicine (Encinitas, Calif.), InnoVision Professional Media, Aug. 2016, www.ncbi.nlm.nih.gov/pmc/articles/PMC4991651/.
Leonard, Jayne. �Hypochlorhydria (Low Stomach Acid): Causes, Symptoms, and Treatment.� Medical News Today, MediLexicon International, 17 July 2018, www.medicalnewstoday.com/articles/322491.php.
MacGill, Markus. �Acid Reflux: Causes, Treatment, and Symptoms.� Medical News Today, MediLexicon International, 13 Nov. 2017, www.medicalnewstoday.com/articles/146619.php.
Ramsay, Philip T, and Aaron Carr. �Gastric Acid and Digestive Physiology.� The Surgical Clinics of North America, U.S. National Library of Medicine, Oct. 2011, www.ncbi.nlm.nih.gov/pubmed/21889024.
Team, Healthline Editorial. �Symptoms of Acid Reflux.� Healthline, 21 June, 2016, www.healthline.com/health/gerd/acid-reflux-symptoms.
Testerman, Traci L, and James Morris. “Beyond the Stomach: An Updated View of Helicobacter Pylori Pathogenesis, Diagnosis, and Treatment.” World Journal of Gastroenterology, Baishideng Publishing Group Inc, 28 Sept. 2014, www.ncbi.nlm.nih.gov/pmc/articles/PMC4177463/.
Wang, Yao-Kuang, et al. �Current Pharmacological Management of Gastroesophageal Reflux Disease.� Gastroenterology Research and Practice, Hindawi Publishing Corporation, 2013, www.ncbi.nlm.nih.gov/pmc/articles/PMC3710614/.

by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gut and Intestinal Health, Health, Wellness
Do you feel:
- Difficulty digesting roughage and fiber
- Indigestion and fullness the last 2-4 hours
- Pain, tenderness, soreness on the left side, under the rib cage
- Nausea or vomiting
- Stool undigested, foul-smelling, mucus-like, greasy or poorly formed
If you are experiencing any of these situations, then you might be experiencing pancreatic digestive disorders.
The Pancreas
The pancreas is a gland organ located in the abdomen. It is part of the digestive system, producing insulin and other vital enzymes and hormones that help break down food. It has an endocrine function, due to releasing juices directly into the bloodstream and has an exocrine function that releases juices into the ducts in the body.
One of its many jobs the pancreas does is that it secretes out enzymes into the small intestine and continues to break down that left in the stomach. Another job is that it produces insulin and secretes it into the bloodstream, where it can regulate the body’s glucose or sugar level. When there is a problem with insulin control in the body, it can lead a person to have diabetes. Other health problems include pancreatitis and pancreatic cancer.
Pancreatitis

Pancreatitis is an inflammation in the pancreas. It occurs when the digestive enzymes become activated while irritating the cells in the pancreas. With repeated damages to the pancreas, it can cause either two forms of pancreatitis, which is acute pancreatitis and chronic pancreatitis. Both are very painful and can form scar tissue in the pancreas, causing it to lose its function. A poorly functioning pancreas can cause digestion problems and diabetes. Here are the conditions that can lead to pancreatitis:
- Abdominal surgery
- Alcoholism
- Certain medications
- Cystic fibrosis
- Gallstones
- Obesity
Chronic Pancreatitis
Chronic pancreatitis is a long-term progressive inflammatory disease in the pancreas that can lead to a permanent breakdown of the structure and function of the pancreas in the body. Studies stated that the most common cause of chronic pancreatitis is long term alcohol abuse, it is thought to account for between 70 to 80 percent for all cases, and significantly it affects more men than women.
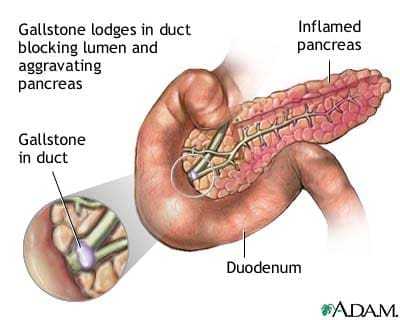
Common signs and symptoms of chronic pancreatitis include:
- Severe upper abdominal pain that is more intense after a meal
- Nausea and vomiting
When the disease progresses, the episodes of pain will become more frequent and more severe to individuals. Some individuals eventually suffer from constant abdominal pain, and as chronic pancreatitis progresses, the ability of the pancreas to produce digestive juices will deteriorate, and the following symptoms appear:
- Smelly and greasy stool
- Bloating
- Abdominal cramps
- Flatulence
- Diabetes
There are numerous complications that an individual can potentially have with chronic pancreatitis. Nutrient malabsorption is one of the most complications since the pancreas is not producing enough digestive enzymes for the body to absorb the nutrients properly, leading to malnutrition. Another possible complication is the development of diabetes, where chronic pancreatitis damages the cells that produce insulin and glucagon to the body. Some individuals will also develop pseudocyst, which is fluid-filled that can form inside or outside the pancreas and can be very dangerous to the body since they can block the essential ducts and blood vessels.
Acute Pancreatitis
Acute pancreatitis is a sudden inflammation of the pancreas. It causes the enzymes to be excessively produced, causing the pancreas gland to be swollen and inflamed. It will make digestion slow down and become painful, making the other body functions be affected as well as making the pancreas be permanently damaged and scarred.
Acute pancreatitis is painful and can develop quickly. It can trigger potentially fatal complications as the mortality rate can range from less than 5 percent to over 30 percent, depending on the severity of the condition and if it reaches to the other organs beyond the pancreas. The most common cause of acute pancreatitis is the production of gallstones in the gallbladder, alcohol misuse, and infections.
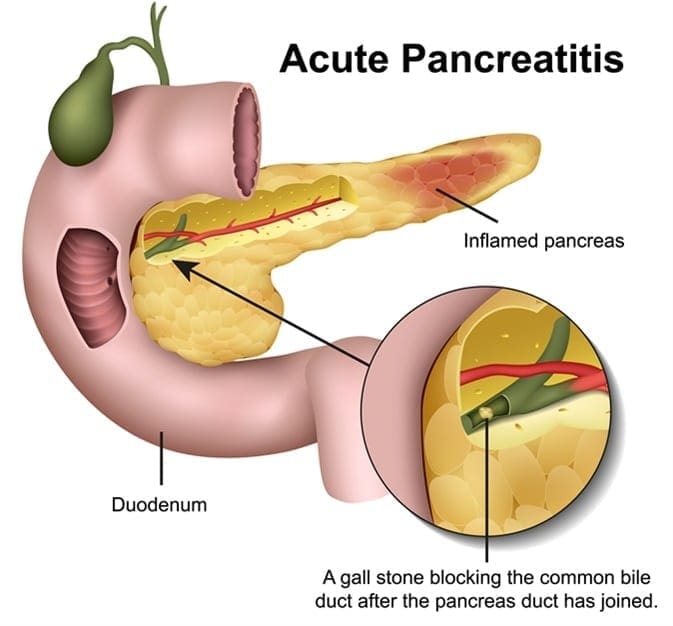
When a person has acute pancreatitis, they feel the pain in the lower abdomen and then feel it more gradually as the pain intensified until it is a constant ache. Other symptoms include:
- Vomiting and nausea
- Diarrhea
- Loss of appetite
- Pain with coughing, vigorous movements, and deep breathing
- Tenderness when the abdomen is touched
- Fever
- Jaundice, yellowish tinge on the skin and the whites of the eyes
Pancreatic Cancer
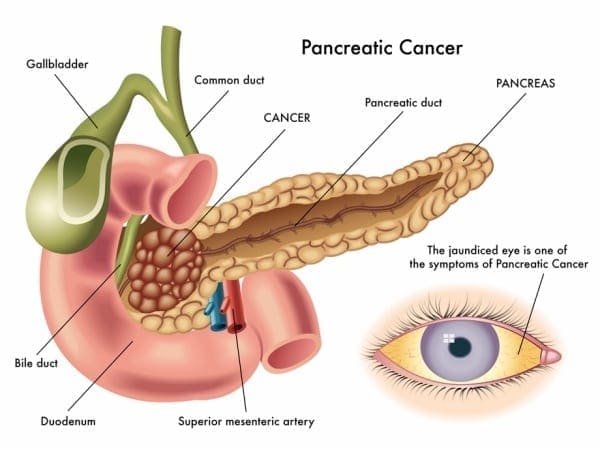
Also known as �the silent disease,” pancreatic cancer happens when uncontrolled cell growth begins to form in a part of the pancreas. Tumors develop and interfere with the way the pancreas works. Pancreatic cancer often shows any symptoms until the later stages, and it can be challenging to manage. The signs and symptoms of pancreatic cancer include:
- Pain in the upper abdomen that radiates to the back
- Unintended weight loss or loss of appetite
- Pale or grey fatty stool
- Jaundice
- New-onset diabetes
- Blood clots
- Depression
- Fatigue
Conclusion
The pancreas is located in the abdomen, and its primary function is to produce insulin and necessary enzymes and hormone to aid the digestion of food. When complications are attacking the pancreas like pancreatic cancer and pancreatitis, it can damage the pancreas to stop producing insulin and can lead to chronic illnesses to spread all over the body. Some products can help support the sugar metabolism that the pancreas creates and offer nutrients and enzymatic cofactors to the gastrointestinal system in the body.
October is Chiropractic Health Month. To learn more about it, check out Governor Abbott�s proclamation on our website to get full details on this historic moment.
The scope of our information is limited to chiropractic, musculoskeletal and nervous health issues as well as functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or chronic disorders of the musculoskeletal system. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900 .
Reference:
Banks, Peter A, et al. �The Management of Acute and Chronic Pancreatitis.� Gastroenterology & Hepatology, Millennium Medical Publishing, Feb. 2010, www.ncbi.nlm.nih.gov/pmc/articles/PMC2886461/.
Bartel, Michael. �Acute Pancreatitis – Gastrointestinal Disorders.� MSD Manual Professional Edition, MSD Manuals, July 2019, www.msdmanuals.com/en-gb/professional/gastrointestinal-disorders/pancreatitis/acute-pancreatitis.
Brazier, Yvette. �Acute Pancreatitis: Symptoms, Treatment, Causes, and Complications.� Medical News Today, MediLexicon International, 19 Dec. 2017, www.medicalnewstoday.com/articles/160427.php.
Brazier, Yvette. �Pancreatic Cancer: Symptoms, Causes, and Treatment.� Medical News Today, MediLexicon International, 23 Oct. 2018, www.medicalnewstoday.com/articles/323423.php.
Colledge, Helen, et al. �Chronic Pancreatitis.� Healthline, 14 Sept. 2017, www.healthline.com/health/chronic-pancreatitis.
Crosta, Peter. �Pancreas: Functions and Disorders.� Medical News Today, MediLexicon International, 26 May 2017, www.medicalnewstoday.com/articles/10011.php.
Felman, Adam. �Chronic Pancreatitis: Treatments, Symptoms, and Causes.� Medical News Today, MediLexicon International, 19 Dec. 2017, www.medicalnewstoday.com/articles/160459.php.
Health Publishing, Harvard. �Acute Pancreatitis.� Harvard Health, July 2019, www.health.harvard.edu/a_to_z/acute-pancreatitis-a-to-z.
Staff, Mayo Clinic. �Pancreatic Cancer.� Mayo Clinic, Mayo Foundation for Medical Education and Research, 9 Mar. 2018, www.mayoclinic.org/diseases-conditions/pancreatic-cancer/symptoms-causes/syc-20355421.
Staff, Mayo Clinic. �Pancreatitis.� Mayo Clinic, Mayo Foundation for Medical Education and Research, 7 Sept. 2019, www.mayoclinic.org/diseases-conditions/pancreatitis/symptoms-causes/syc-20360227.
by Dr Alex Jimenez DC, APRN, FNP-BC, CFMP, IFMCP | Functional Medicine, Gastro Intestinal Health, Gut and Intestinal Health, Health, Wellness
Do you feel:
- Excessive belching, burping or bloating
- A sense of fullness during and after meals
- Gas immediately after a meal
- Offensive breath
- Difficulty digesting proteins and meats
If you are experiencing any of these situations, then you might be experiencing some stomach digestive disorders.
The Stomach
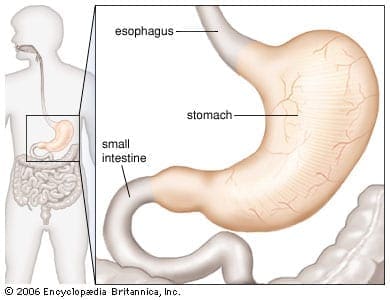
The human stomach�s core function is to aid food to digest when an individual eats. The four critical components of the gastric digestive function are:
- A reservoir capacity
- Acid secretion
- Enzyme secretion
- Gastrointestinal motility
These four components help the stomach function properly in the digestive system and help the body absorb essential nutrients and are responsible for getting rid of waste out of the body. Any disorders like GERD, gallstones, and Crohn’s disease are a few of the many illnesses that can affect not only the stomach but the entire digestive system. It can cause a person to feel discomfort and can be long term if the individual has not treated it.
GERD
GERD or gastroesophageal reflux disease when the contents from the stomach move up into the esophagus, causing acid reflux. Researchers at the NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases) stated that about 20% of individuals are affected by GERD, if it is left untreated, it can sometimes cause serious complications.
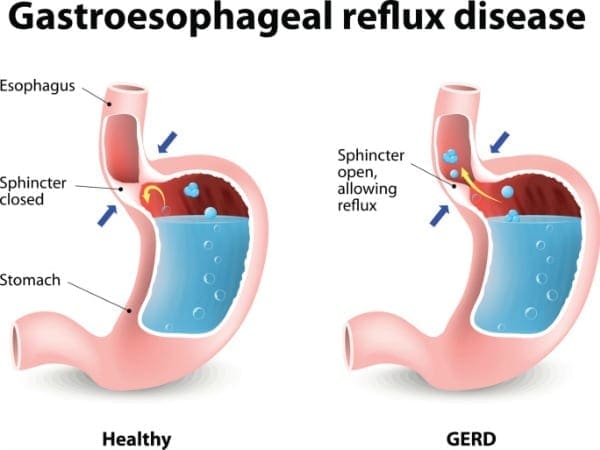
One of the main symptoms that GERD causes is heartburn. Heartburn is a discomfort feeling that is felt from behind the breastbone as a burning sensation. It tends to get worse on a person if they lay down, bend over, after eating food. Not all individuals with GERD experiences heartburn, there are other possible symptoms such as:
- Chest pains
- Difficulty swallowing
- Bad breath
- The sensation of a lump in the throat
- Sour taste in the mouth
- Respiratory problems
- Tooth decay
Gallstones
Gallstones are hardened deposits of digestive fluids that can form in the gallbladder. The gallbladder is a small, pear-shaped organ that’s located on the right side of the abdomen, just beneath the liver. It also holds bile fluid that releases into the small intestines. Gallstones can range in sizes from as small as sand to as large as a golf ball. According to Harvard Health Publications, about 80% of gallstones are made of cholesterol, while the other 20% is made up of calcium salts and bilirubin.
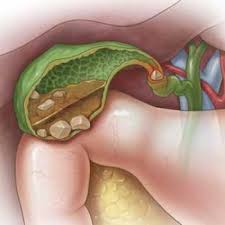
Gallstones can lead to pain in the upper right abdomen. An individual may start to feel gallbladder pains when they eat foods that are high in fat, especially fried foods. Furthermore, if the pain continues, it may lead to an inflamed gallbladder or cholecystitis. They may also experience symptoms like:
- Pain on the right-hand side of the body, just below the ribs
- Back pain between the shoulder blades
- Pain in the right shoulder
- Nausea
- Dark urine
- Clay-colored stool
- Stomach pain
Researchers stated that some people develop the chemical imbalance in their gallbladders causes gallstones while others do not. Gallstones are more common among people with obesity, and studies revealed that women can develop gallstones and may require surgery to remove them.
Crohn�s Disease
Crohn�s disease is an inflammatory disease. It causes inflammation in the body’s digestive tract and can cause several chronic illnesses. Inflammation caused by Crohn’s disease can be in different areas of the digestive tract in different people. The inflammation often spreads deep into the layers of the affected bowel tissue, causing pain, and sometimes lead to life-threating complications.
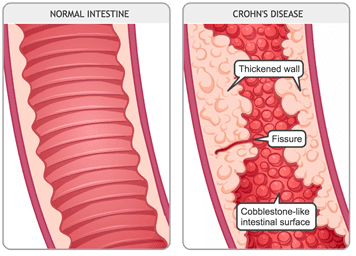
Crohn�s disease symptoms can vary depending on which part of the gut is affected in the body. Specific symptoms can often develop gradually and become worse over time, and it is rare for the symptoms of Crohn’s disease to develop suddenly and dramatically. The symptoms of Crohn’s disease include:
- Pain
- Ulcers in the gut
- Mouth ulcers
- Diarrhea
- A fever
- Fatigue
- Loss of appetite
- Rectal bleeding and anal fissures
- Anemia
The exact causes of Crohn’s disease are still unclear, but researchers theorized that it stems from an abnormal reaction in the immune system. The theory stated that the immune system attacks food, good bacteria, and beneficial substances as if they are unwanted substances. During the attack, the body’s white cells start building up in the lining of the gut and triggers inflammation. It is still unclear whether the abnormal immune system causes Crohn’s disease, but there are environmental factors that can increase the risk of inflammation.
Conclusion
The stomach’s primary function is to digest the food that a person consumes. Four components help aid the stomach to function correctly. When the stomach is dealing with chronic illnesses like Crohn’s disease, gallstones, and GERD, it can lead to inflammation on the intestinal barriers. When it is left untreated, it can lead to life long complicated problems in the body. Some products can help aid the stomach digestion as they help support the gastrointestinal system as well.
October is Chiropractic Health Month. To learn more about it, check out Governor Abbott�s bill on our website to get full details on this historic moment.
The scope of our information is limited to chiropractic, musculoskeletal and nervous health issues as well as functional medicine articles, topics, and discussions. We use functional health protocols to treat injuries or chronic disorders of the musculoskeletal system. To further discuss the subject matter above, please feel free to ask Dr. Alex Jimenez or contact us at 915-850-0900 .
References:
AAAS, EurekAlert. �A Bulging Midriff Roughly Doubles Women’s Chances of Gallstone Surgery.� EurekAlert!, 13 Feb. 2006, www.eurekalert.org/pub_releases/2006-02/bsj-abm021006.php.
Brazier, Yvette. �Cholecystitis: Symptoms, Causes, Diagnosis, and Treatment.� Medical News Today, MediLexicon International, 22 Jan. 2018, www.medicalnewstoday.com/articles/172067.php.
Brazier, Yvette. �Crohn’s Disease: Symptoms, Diet, Treatment, and Causes.� Medical News Today, MediLexicon International, 11 Jan. 2019, www.medicalnewstoday.com/articles/151620.php.
Editorial Team, Healthline, and Heather Cruickshank. �Everything You Need to Know About Acid Reflux and GERD.� Healthline, 7 Dec. 2018, www.healthline.com/health/gerd.
Holland, Kimberly. �Understanding Crohn’s Disease.� Healthline, 2 May, 2019, www.healthline.com/health/crohns-disease.
MacGill, Markus. �GERD: Symptoms, Causes, and Treatment.� Medical News Today, MediLexicon International, 18 Jan. 2018, www.medicalnewstoday.com/articles/14085.php.
Macon, Brindles Lee, et al. �Understanding Gallstones: Types, Pain, and More.� Healthline, 1 June, 2017, www.healthline.com/health/gallstones.
O’Connor, Anthony, and Colm O’Mor�in. �Digestive Function of the Stomach.� Digestive Diseases (Basel, Switzerland), U.S. National Library of Medicine, 2014, www.ncbi.nlm.nih.gov/pubmed/24732181.
Publishing, Harvard Health. �What to Do about Gallstones.� Harvard Health, 2011, www.health.harvard.edu/womens-health/what-to-do-about-gallstones.
Staff, Mayo Clinic. �Crohn’s Disease.� Mayo Clinic, Mayo Foundation for Medical Education and Research, 13 Sept. 2019, www.mayoclinic.org/diseases-conditions/crohns-disease/symptoms-causes/syc-20353304.
Staff, Mayo Clinic. �Gallstones.� Mayo Clinic, Mayo Foundation for Medical Education and Research, 8 Aug. 2019, www.mayoclinic.org/diseases-conditions/gallstones/symptoms-causes/syc-20354214.
Unknown, Unknown. �Definition & Facts for GER & GERD.� National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Department of Health and Human Services, 1 Nov. 2014, www.niddk.nih.gov/health-information/digestive-diseases/acid-reflux-ger-gerd-adults/definition-facts.






































