Pain Modulation Pathway Mechanisms in El Paso, TX
Most, if not all, ailments of the body trigger pain. Pain is interpreted and sensed in the brain. Pain is modulated by two key types of drugs which operate on the brain: analgesics and anesthetics. The term analgesic refers to a medication that relieves pain without loss of consciousness. The expression central anesthesia refers to a medication that depresses the CNS. It’s distinguished by the lack of all perception of sensory modalities, for instance, loss of consciousness without loss of critical functions.
Opiate Analgesia (OA)
The most successful clinically used drugs for producing temporary analgesia and relief from pain are the opioid family, which includes morphine, and heroin. There are currently no additional powerful pain therapeutic options to opiates. Several side effects caused by opiate use include tolerance and drug dependence or addiction. In general, these drugs modulate the incoming pain information in the spine and central nervous system, in addition to relieve pain temporarily, and can also be called opiate producing analgesia (OA). Opiate antagonist is a drug that antagonizes the opioid effects, such as naloxone or maltroxone, etc.. They are competitive antagonists of opiate receptors. However, the brain has a neuronal circuit and endogenous substances which modulate pain.
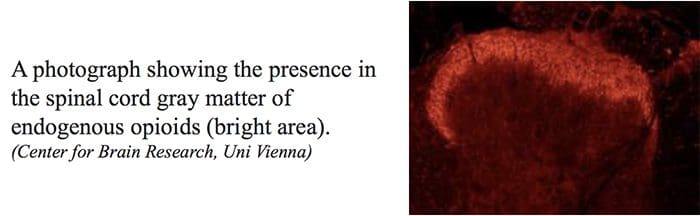
Endogenous Opioids
Opioidergic neurotransmission is located throughout the brain and spinal cord and is believed to influence many functions of the central nervous system, or CNS, such as nociception, cardiovascular functions, thermoregulation, respiration, neuroendocrine functions, neuroimmune functions, food consumption, sexual activity, competitive locomotor behaviour as well as memory and learning. Opioids exert marked effects on mood and motivation and produce a sense of euphoria.
Three classes of opioid receptors are identified: ?-mu, ?-delta and ?-kappa. All 3 classes are widely dispersed in the brain. The genes encoding each one of these have been cloned and found to function as members of the G protein receptors. Moreover, three major types of endogenous opioid peptides that interact with the above opiate receptors have been recognized in the central nervous system, including, ?-endorphins, enkephalins and the dynorphins. These 3 opioid peptides are derived from a large protein receptor by three different genes, such as the proopiomelanocortin, or POMC, gene, the proenkephalin gene and the prodynorphin gene.�The opioid peptides modulate nociceptive input in two ways: first, they block neurotransmitter release by inhibiting Ca2+ influx into the presynaptic terminal, or second, they open potassium channels, which hyperpolarizes neurons and inhibits spike activity. They act on various receptors within the brain and spinal cord.
Enkephalins are considered the putative ligands for the ? receptors, ? endorphins for its ?-receptors, and dynorphins for the ? receptors. The various types of opioid receptors are distributed differently within the peripheral and central nervous system, or CNS. There’s evidence for functional differences in these receptors in various structures. This explains why many undesirable side effects occur after opiate treatments. For instance, mu (?) receptors are widespread in the brain stem parabrachial nuclei, where a respiratory center and inhibition of these neurons may cause what’s known as respiratory depression.
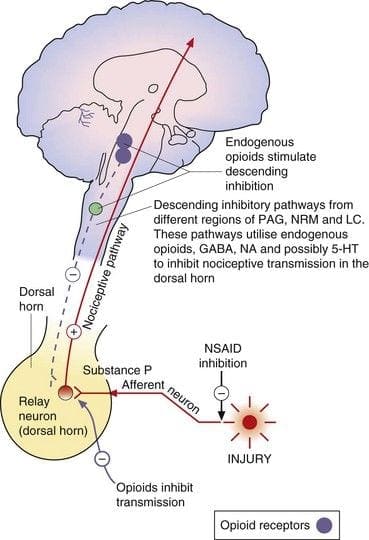
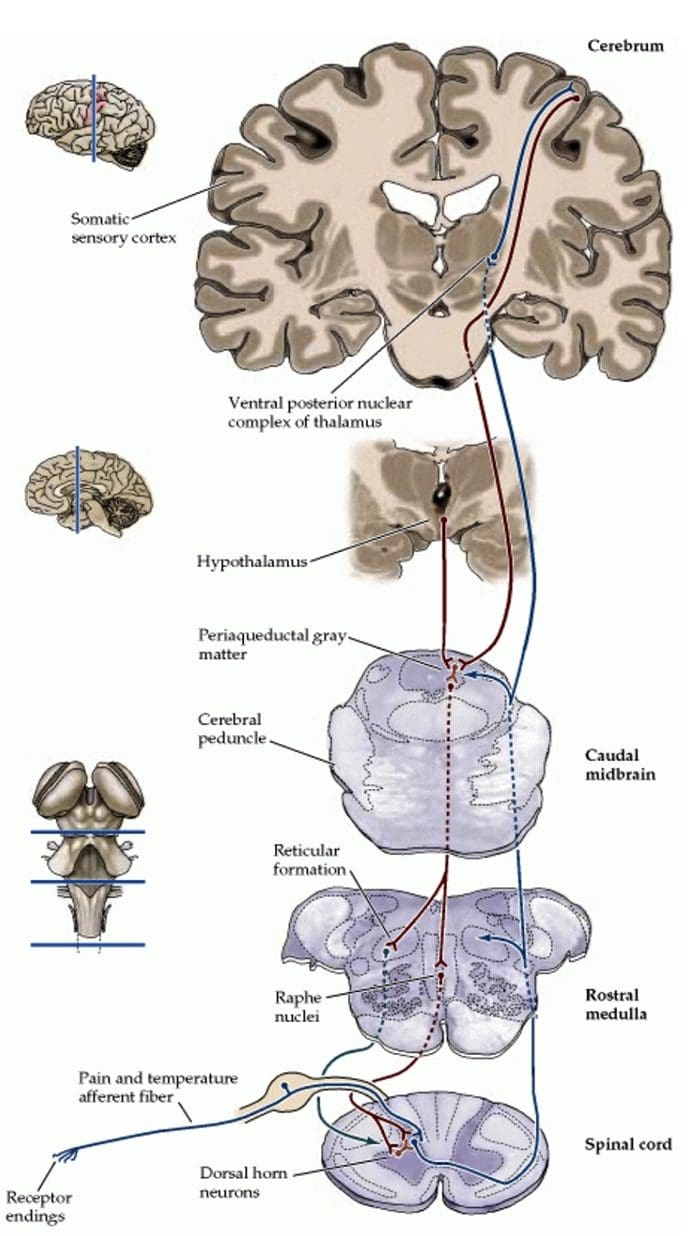
Central or peripheral terminals of nociceptive afferent fibers feature opiate receptors in which exogenous and endogenous opioids could act to modulate the capability to transmit nociceptive information. Additionally, high densities of opiate receptors are found in periaqueductal gray, or PAG, nucleus raphe magnus, or NRM, and dorsal raphe, or DR, from the rostral ventral medulla, in the spinal cord, caudate nucleus, or CN, septal nucleus, hypothalamus, habenula and hippocampus.�Systemically administered opioids at analgesic dosages activate spinal and supraspinal mechanisms via ?, ?, and ? type opioid receptors and regulate pain signals to modulate symptoms.
Neuronal Circuits and Pain Modulation
For many decades it was suggested that somewhere in the central nervous system there is a circuit which can modulate incoming pain details. The gate control theory and the ascending/descending pain transmission system are two suggestions of such a circuit. Below, we will discuss both in further detail.
Gate Control Theory
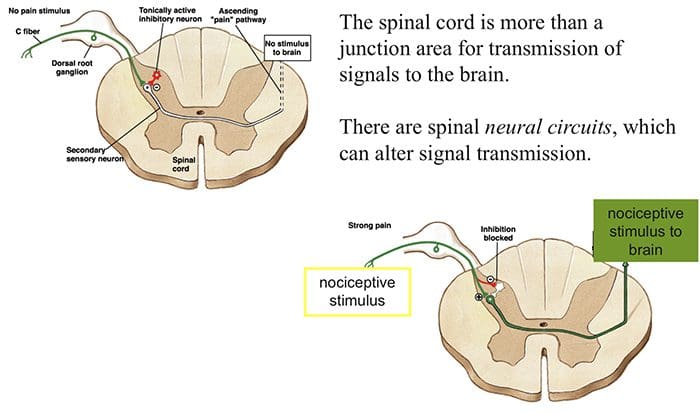
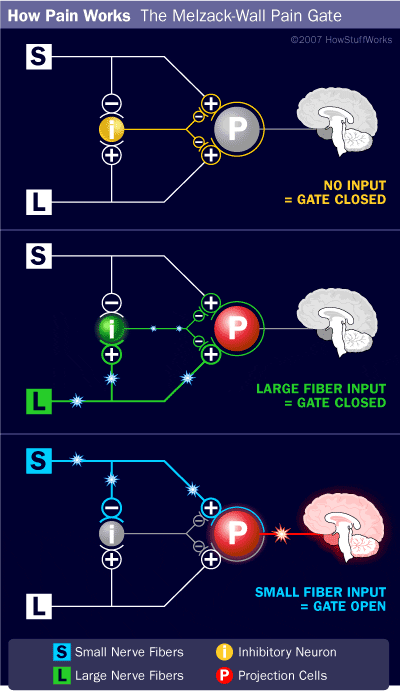
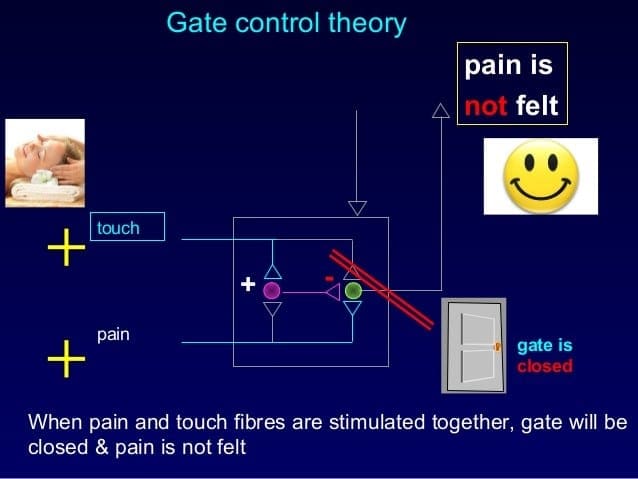
The initial pain modulatory mechanism known as the gate control theory, has been proposed by Melzack and Wall in the mid 1960’s. The notion of the gate control theory is that non-painful input closes the gates to painful input, which results in avoidance of the pain sensation from travel into the CNS, for example, non-noxious input, or stimulation, suppresses pain.
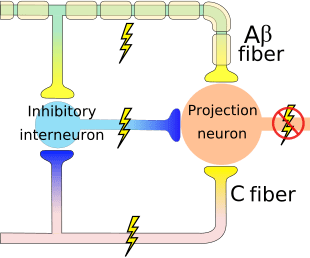
The theory implies that collaterals of the large sensory fibers carrying cutaneous sensory input activate inhibitory interneurons, which inhibit and regulate pain transmission data carried from the pain fibers. Non-noxious input inhibits pain, or sensory input, and closes the gate to noxious input. The gate control theory demonstrates that in the spinal cord level, non-noxious stimulation will create presynaptic inhibition on dorsal root nociceptor fibers that synapse on nociceptors spinal neurons (T). This presynaptic inhibition will also prevent incoming noxious information from reaching the CNS, for example, it will shut the gate to incoming toxic information.
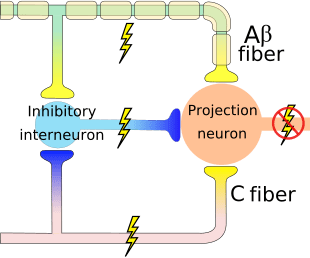
The gate control theory was the rationale for the idea behind the production and utilization of the transcutaneous electrical nerve stimulation, or TENS, for pain relief. In order to be effective, the TENS unit generates two different present frequencies below the pain threshold that can be taken by the patient. This process has found a degree of achievement in chronic pain treatment.
Pain Modulation: Gate Control Theory
Stimulation Produced Analgesia (SPA)
Evidence for an inherent analgesia system was found by intracranial electrical stimulation of certain discrete brain regions. These areas would be the periaqueductal gray, or PAG, and nucleus raphe magnus, or NRM, dorsal raphe, or DR, caudate nucleus, or CN, septal nucleus, or Spt, along with other nuclei. Such stimulation or sensory signals, inhibits pain, making analgesia without behavioral suppression, while the touch, temperature and pressure sensation stays intact. According to research studies, SPA, or stimulation produced analgesia, is more pronounced and continues for a longer period of time after stimulation in humans than in experimental animals. Additionally, during SPA, the subjects, however, still respond to nonpainful stimulation like temperature and touch within the circumscribed region of analgesia. The most effective CNS, or central nervous system regions for SPA to occur, would be in the PAG and the raphe nuclei, or RN.
Electrical stimulation of PAG or NRM inhibits spinal thalamic cells, or spinal neurons that project monosynaptically to the thalamus, in laminae I, II and V to ensure the noxious information from the nociceptors which are ultimately modulated in the level of the spinal cord. Furthermore, PAG has neuronal connections to the nucleus raphe magnus, or NRM.
The activity of the PAG most likely occurs by activation of the descending pathway from NRM and likely also by activation of ascending connections acting on greater subcortical levels of the CNS. In addition, electric stimulation of PAG or NRM produces behavioral analgesia, or stimulation produced analgesia. Stimulation produced analgesia, or SPA causes the release of endorphins which can be blocked by the opiate antagonist naloxone.
During PAG and/or RN stimulation, serotonin, also medically referred to as 5-HT, can also be discharged from ascending and descending axons from subcortical nuclei, in spinal trigeminal nuclei and in the spinal cord. This release of 5-HT modulates and regulates pain transmission by inhibiting or blocking incoming neural action. Depletion of 5-HT by electrical lesion of the raphe nuclei or with a neurotoxic lesion made by local injection of a chemical agent such as parachlorophenylalanine, or PCPA, results in blocking the power of opiate, both intracranial and systemic, as well as that of electrical stimulation in order to produce analgesia.
To confirm if the electric stimulation produced analgesia via the release of opiate and dopamine, then the region is locally microinjected with morphine or 5-HT. All these microinjections ultimately create analgesia. These processes also provide a way of identifying brain areas related to pain suppression and assist to produce a map of pain centers. The most effective way of producing opiate analgesia, or OA, is by intracerebral injection of morphine into the PAG.
The PAG and RN as well as other brain structures in which analgesia is produced, are also rich in opiate receptors. Intracerebral opioid administration produced analgesia and SPA can be blocked by systemic or from local microinjections of naloxone, the morphine antagonist, into the PAG or RN. For that reason, it’s been suggested that the two, both OA and SPA, operate by a frequent mechanism.
If OA and SPA behave through the same intrinsic system, then the hypothesis that opiates activate a pain-suppression mechanism is much more likely. As a matter of fact, current evidence suggests that microinjections of an opiate into the PAG activate an efferent brainstem system which inhibits pain transmission at segmental spinal cord levels. These observations imply that analgesia elicited from the periaqueductal gray, or PAG, demands a descending pathway into the spinal cord.

Dr. Alex Jimenez’s Insight
Pain modulation occurs through the process of electrical brain stimulation which occurs due to the activation of descending inhibitory fibers, which regulate or inhibit the input and output of certain neurons. What has been described as opioid and serotonergic antagonists, is believed to reverse both local opiate analgesia and brain-stimuli generated analgesia. The sensory signals or impulses in the central nervous system are ultimately controlled by both ascending and descending inhibitory systems, utilizing endogenous opioids or other endogenous substances, such as serotonin as inhibitory mediators. Pain is a complex perception which can also be influenced by a variety of other factors, including emotional state.
Mechanisms of Pain Modulation
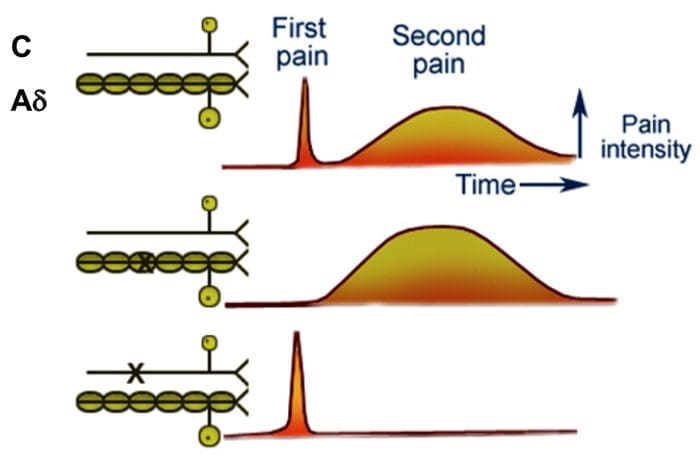
Ascending and Descending Pain Suppression Mechanism
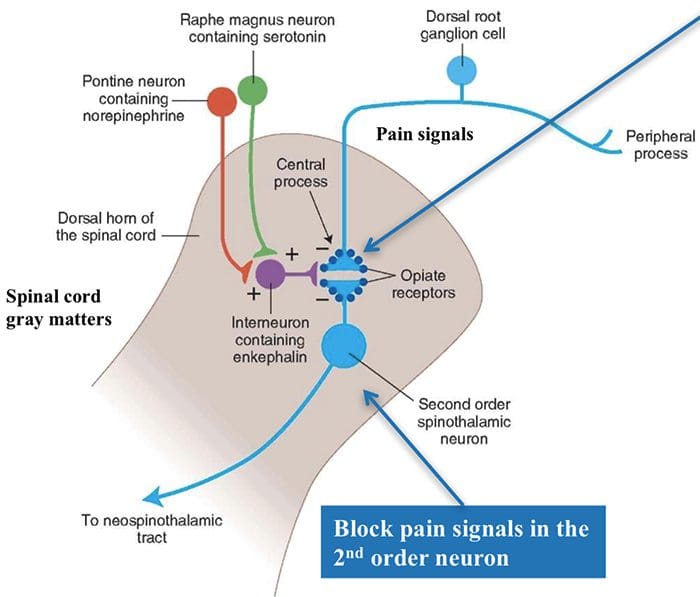
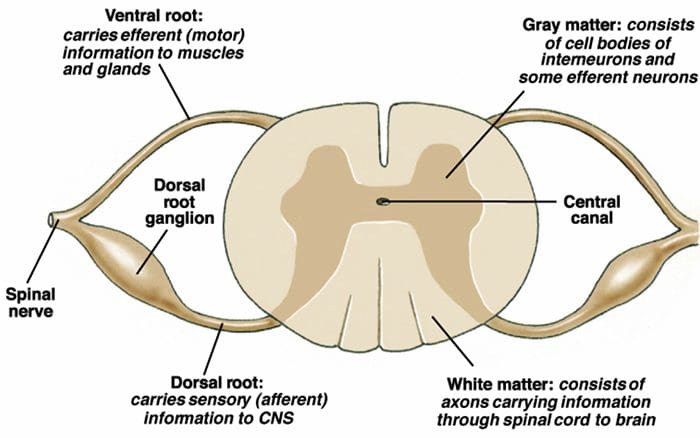
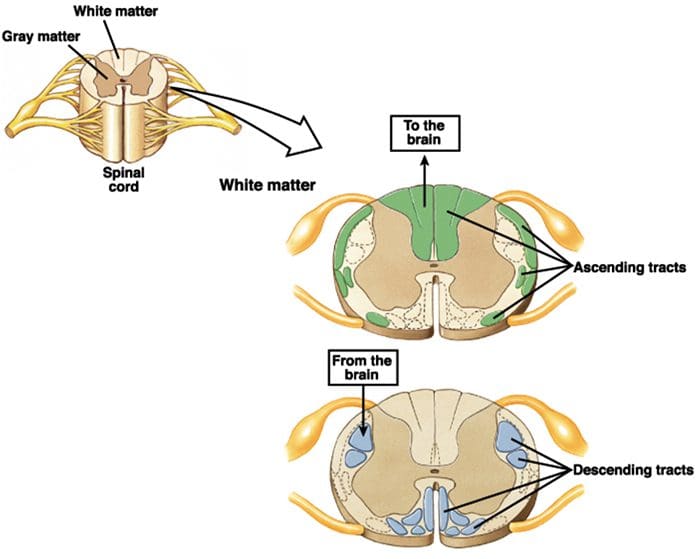
The primary ascending pain fibers, such as the A ? and C fibers, reach the dorsal horn of the spinal cord from peripheral nerve areas in order to innervate the nociceptor neurons in Rexed laminae I & II. Cells from Rexed lamina II make synaptic connections in Rexed layers IV to VII. Cells, particularly within laminae I and VII of the dorsal horn, give rise to ascending spinothalamic tracts. In the spinal level, opiate receptors are located in the presynaptic endings of their nocineurons and in the interneural level layers IV to VII from the dorsal horn.
Activation of opiate receptors at the interneuronal level produces hyperpolarization of the neurons, which lead to the the inhibition of activation as well as the release of substance P, a neurotransmitter involved in pain transmission, thus preventing pain transmission. The circuit which consists of the periaqueductal gray, or PAG, matter in the upper brain stem, the locus coeruleus, or LC, the nucleus raphe magnus, or the NRM, and the nucleus reticularis gigantocellularis, or Rgc,� leads to the descending pain suppression pathway, which inhibits incoming pain data at the spinal cord level.
As stated before, opioids interact with the opiate receptors in distinct central nervous system levels. These opiate receptors are the normal target regions for hormones and endogenous opiates, such as the endorphins and enkephalins. Due to binding at the receptor in subcortical websites, secondary changes which result in some change in the electrophysiological properties of the neurons and regulation of their ascending pain information.
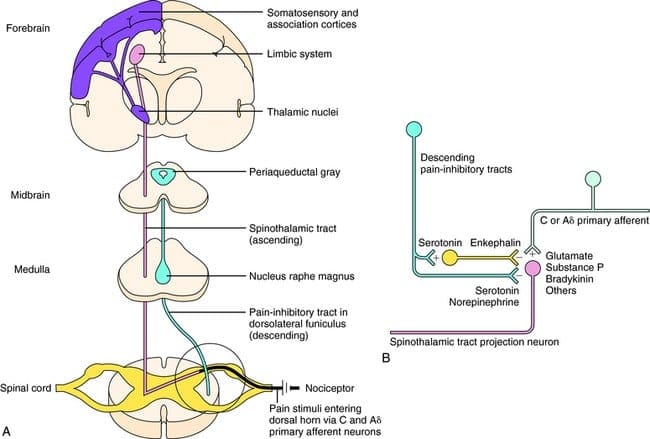
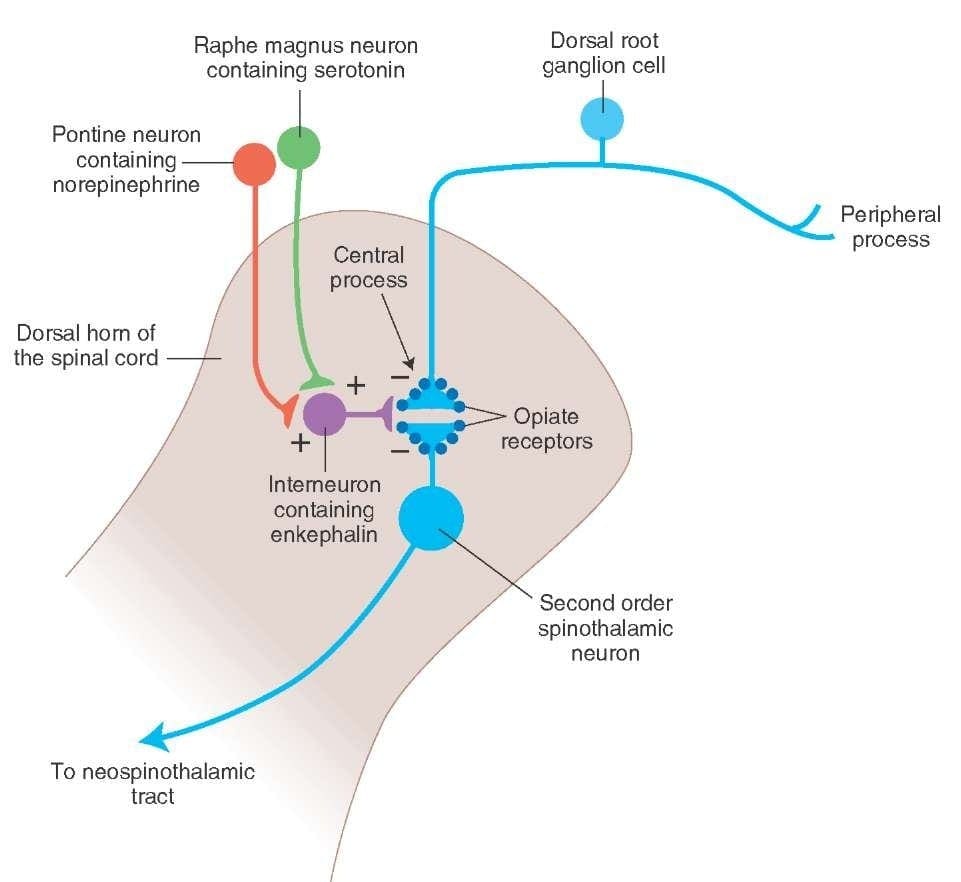
What activates the PAG to exert its consequences? It was discovered that noxious stimulation triggers neurons in the nucleus reticularis gigantocellularis, or RGC. The nucleus Rgc innervates both PAG and NRM. The PAG sends axons into the NRM, and nerves in the NRM send their axons to the spinal cord. Additionally, bilateral dorsolateral funiculus, or DLF, lesions, referred to as DLFX, block the analgesia produced by both electrical stimulation and by microinjection of opiates directly into the PAG and NRM, but they just attenuate the systemic analgesic effects of opiates. These observations support the hypothesis that discrete descending pathways from the DLF are necessary for both OA and SPA.
The DLF is comprised of fibers originating from several brainstem nuclei, which can be serotonergic, or 5-HT, from nerves located inside the nucleus raphe magnus, or NRM; dopaminergic neurons originating from ventral tegmental area, or VTA, and adrenergic neurons originating from the locus coeruleus, or LC. These descending fibers suppress noxious input in the nociceptive spinal cord neurons in laminae I, II, and V.
Opiate receptors have also been discovered in the dorsal horn of the spinal cord, chiefly in Rexed laminae I, II, and V, and such spinal opiate receptors mediate inhibitory effects on dorsal horn neurons transmitting nociceptive information. The action of morphine seems to be exerted equally in the spinal cord and brainstem nuclei, including the PAG and NRM. Systemic morphine acts on both brain stem and spinal cord opiate receptors to produce analgesia. Morphine binds the brainstem opiate receptors, which triggers the brainstem descending serotonergic pathway into the spinal cord as well as the DLF, and these have an opioid-mediated synapse at the level of the spinal cord.
This observation demonstrates that noxious stimuli, instead of non-noxious stimulus, determine the gate control theory, which are critical for the activation of the descending pain modulation circuit where pain inhibits pain via the descending DLF pathway. In addition, there are ascending connections in the PAG and the raphe nuclei into the PF-CM complex. These thalamic regions are a part of the ascending pain modulation at the diencephalon degree.
Stress Induced Analgesia (SIA)
Analgesia may be produced in certain stressful circumstances. Exposure to many different stressful or painful events generates an analgesic response. This phenomenon is known as stress induced analgesia, or SIA. Stress induced analgesia has been believed to give insight into the physiological and psychological factors that trigger endogenous pain control and opiate systems. By way of instance, soldiers injured in battle or athletes hurt in sports sometimes report that they don’t feel pain or discomfort during the battle or game, nevertheless, they will go through the pain afterwards once the specific situation has stopped. It’s been demonstrated in animals that electrical shocks cause stress-induced analgesia. Based on these experiments, it is assumed that the pressure the soldiers and the athletes experienced suppressed the pain which they would later experience.
It’s believed that endogenous opiates are produced in response to stress and inhibit pain by triggering the midbrain descending system. Furthermore, some SIA exhibited cross tolerance with opiate analgesia, which indicates that this SIA is mediated via opiate receptors. Experiments using different parameters of electrical shock stimulation demonstrate such stress induced analgesia and some of those anxieties that produce analgesia could be blocked by the opioid antagonist naloxone, whereas others were not blocked by naloxone. In conclusion, these observations lead to the decision that both opiate and non-opiate forms of SIA exist.
Somatovisceral Reflex
The somatovisceral reflex is a reflex in which visceral functions are activated or inhibited by somatic sensory stimulation. In experimental animals, both noxious and innocuous stimulation of somatic afferents are proven to evoke reflex changes in sympathetic efferent activity and, consequently, effector organ function. These phenomena have been shown in such regions as the gastrointestinal tract, urinary tract, adrenal medulla, lymphatic cells, heart and vessels of the brain and peripheral nerves.
Most frequently, incisions are elicited experimentally by stimulation of cutaneous afferents, even though some work has also been conducted on muscle and articular afferents, including those of spinal cells. The ultimate responses will represent the integration of multiple tonic and reflex influences and might exhibit laterality and segmental trends as well as variable excitability in line with the afferents involved. Given the complexity and multiplicity of mechanisms involved in the last expression of the reflex response, attempts to extrapolate to clinical situations should most likely be conducted in favor of further systematic physiological studies.
The scope of our information is limited to chiropractic as well as to spinal injuries and conditions. To discuss the subject matter, please feel free to ask Dr. Jimenez or contact us at 915-850-0900 .
Curated by Dr. Alex Jimenez
Additional Topics: Sciatica
Sciatica is medically referred to as a collection of symptoms, rather than a single injury and/or condition. Symptoms of sciatic nerve pain, or sciatica, can vary in frequency and intensity, however, it is most commonly described as a sudden, sharp (knife-like) or electrical pain that radiates from the low back down the buttocks, hips, thighs and legs into the foot. Other symptoms of sciatica may include, tingling or burning sensations, numbness and weakness along the length of the sciatic nerve. Sciatica most frequently affects individuals between the ages of 30 and 50 years. It may often develop as a result of the degeneration of the spine due to age, however, the compression and irritation of the sciatic nerve caused by a bulging or herniated disc, among other spinal health issues, may also cause sciatic nerve pain.

EXTRA IMPORTANT TOPIC: Chiropractor Sciatica Symptoms



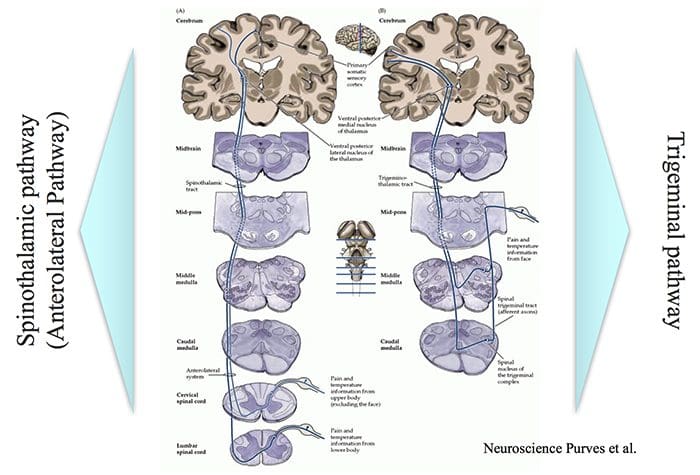
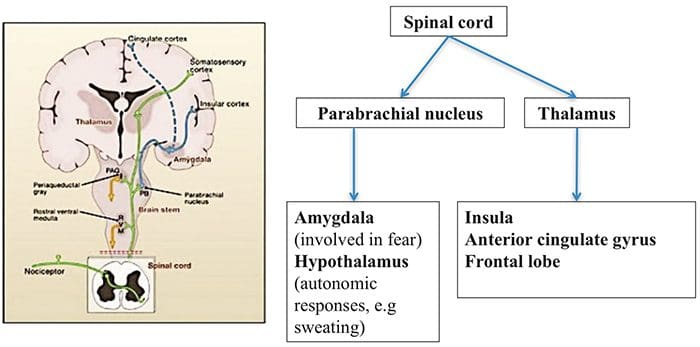 Brain Areas Involved In Processing Of Nociceptive Signals
Brain Areas Involved In Processing Of Nociceptive Signals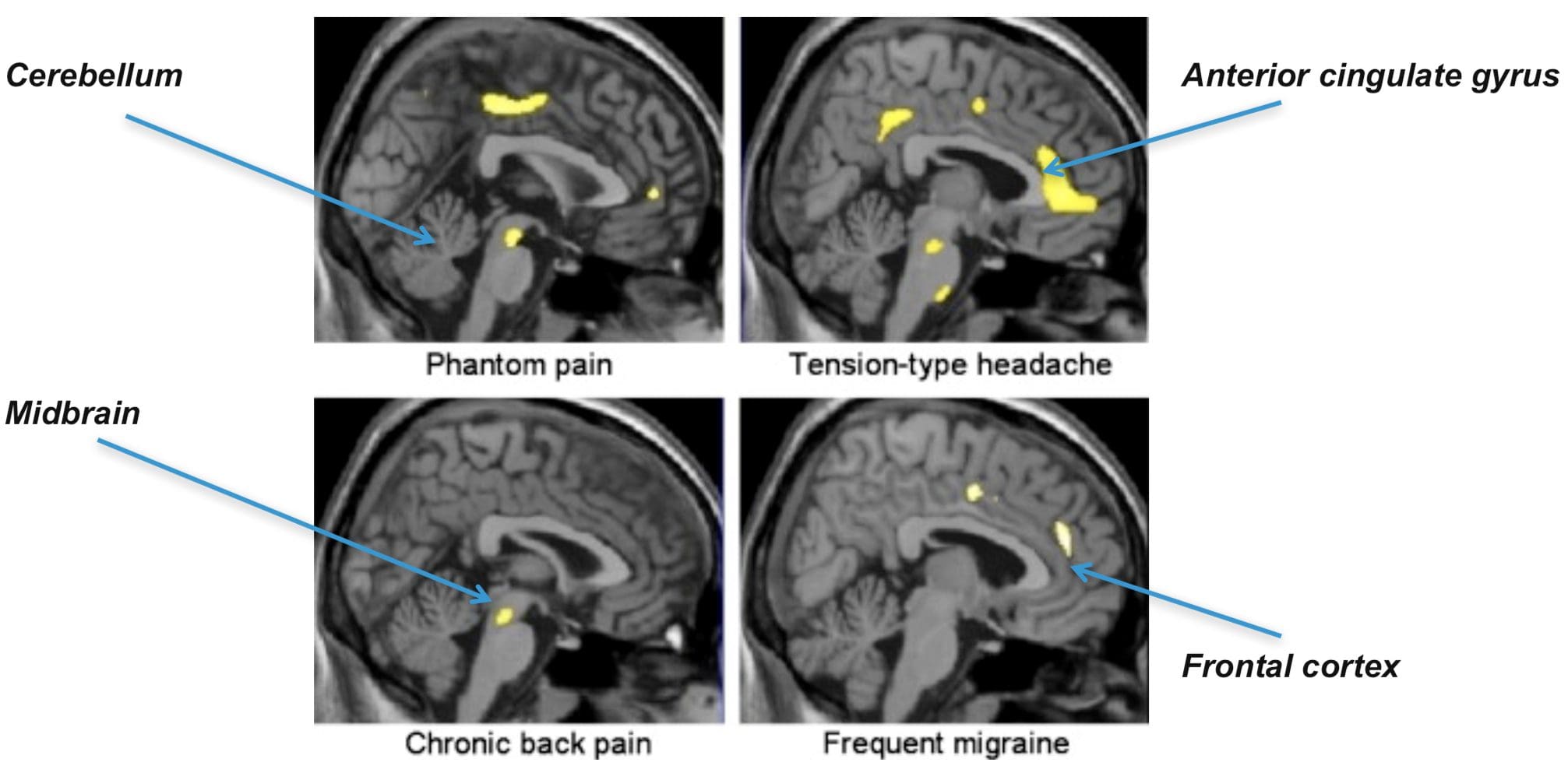 The Anterior Cingulate & Insula Cortex Are Activated In Human Subjects
The Anterior Cingulate & Insula Cortex Are Activated In Human Subjects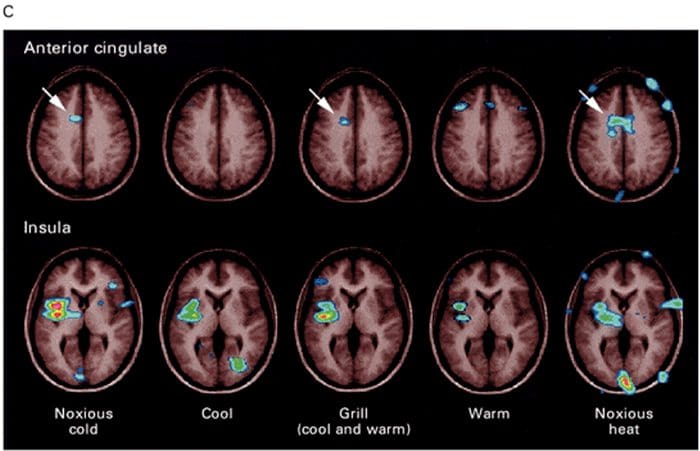
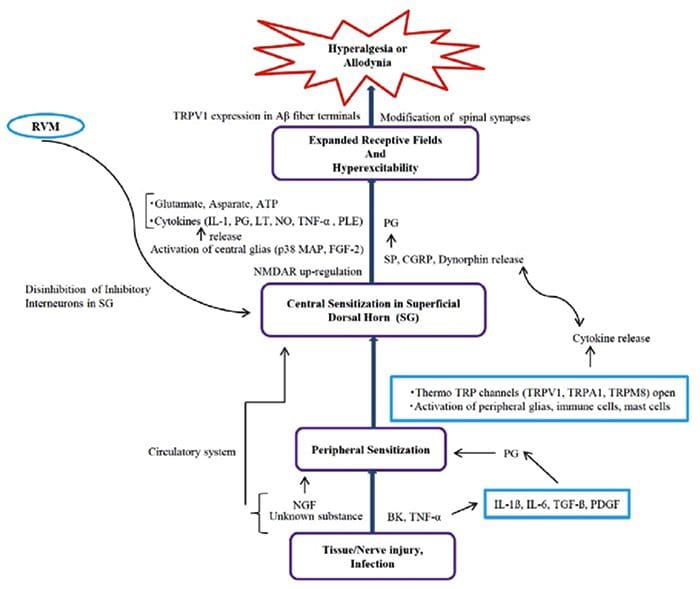
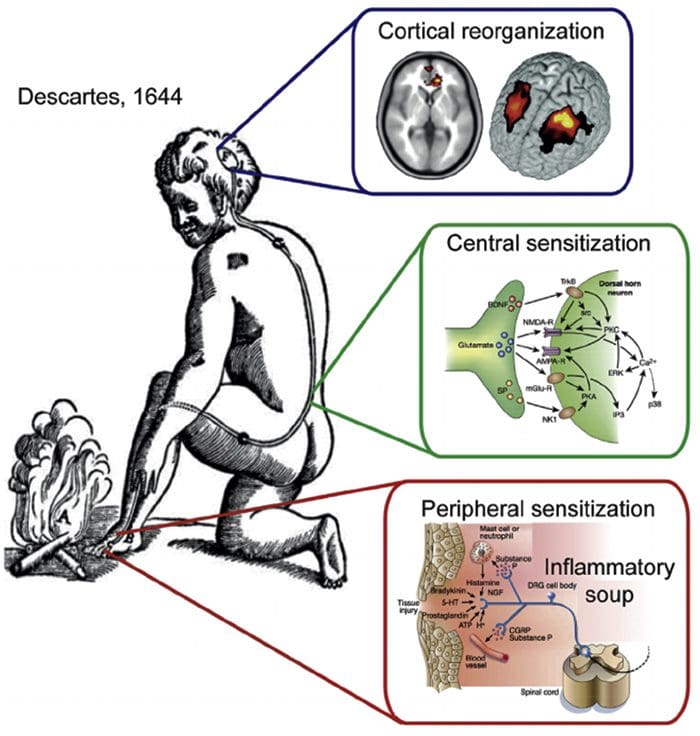
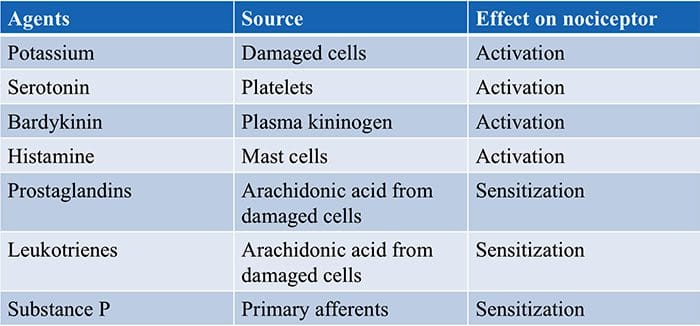
 Inflammatory Soup – Hyperalgesia
Inflammatory Soup – Hyperalgesia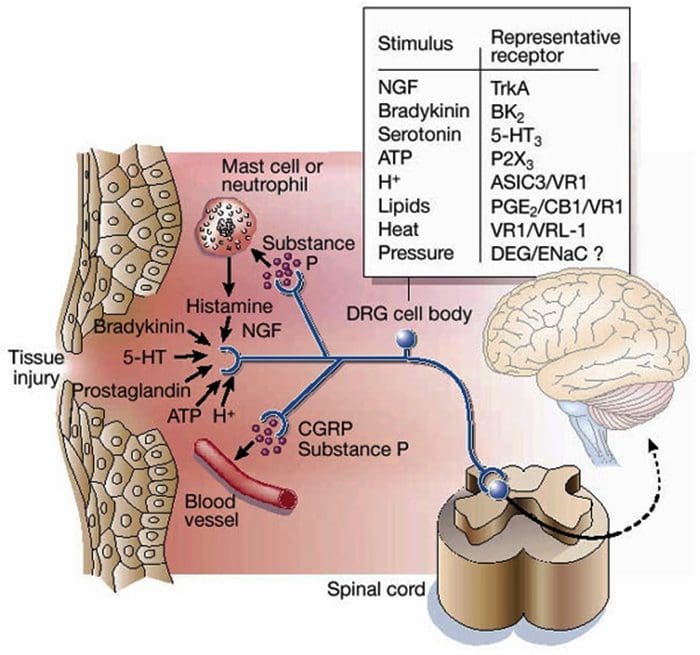
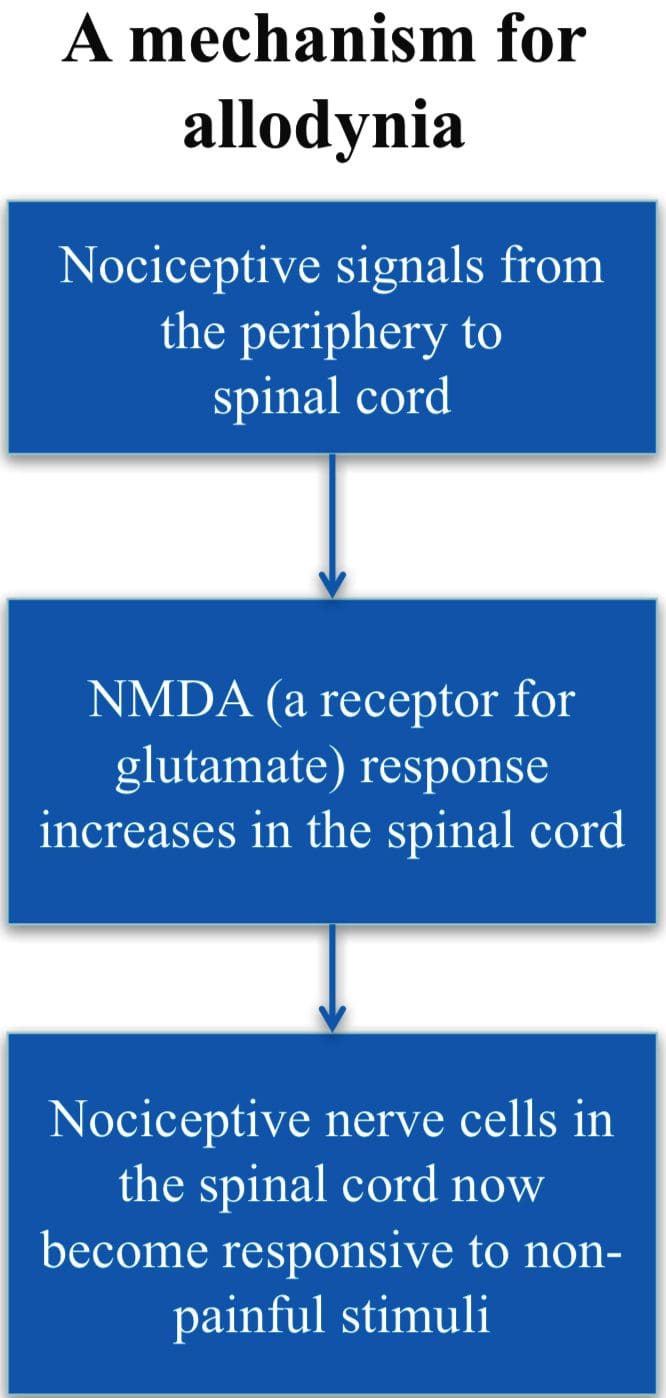 Gate Control Theory of Pain
Gate Control Theory of Pain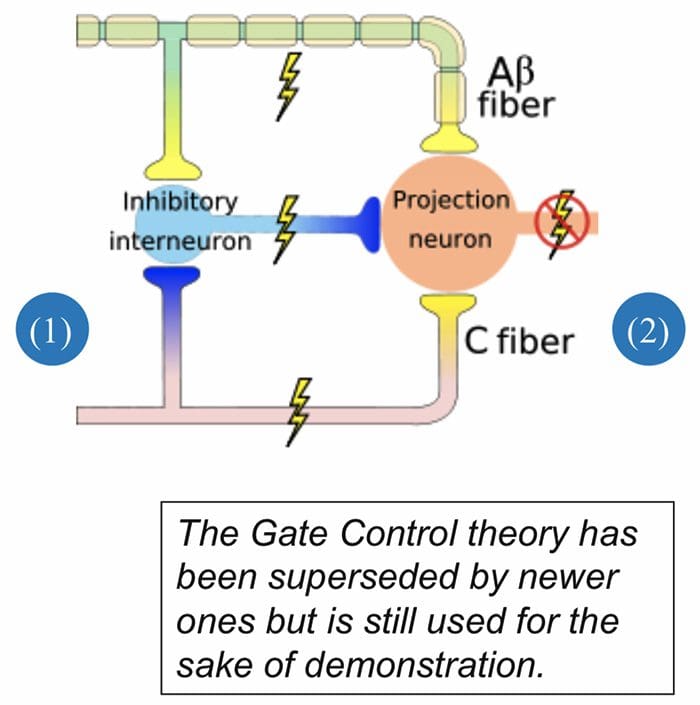
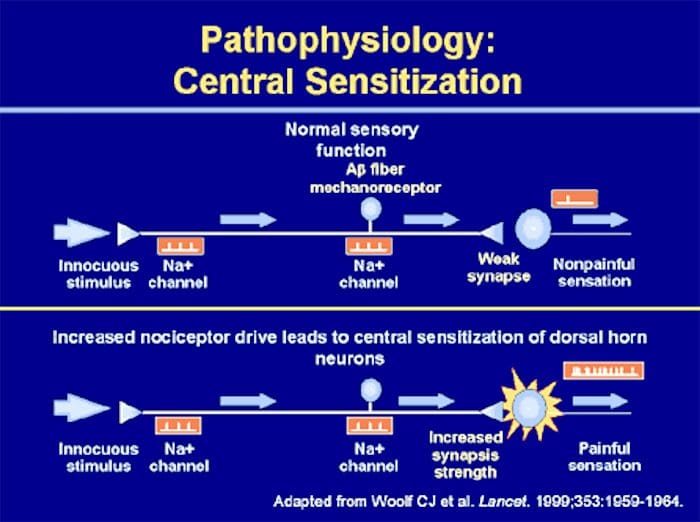
 Abnormalities Of Pain System
Abnormalities Of Pain System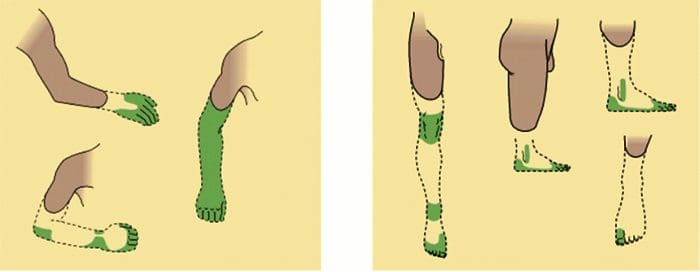 Peripheral Sensitization
Peripheral Sensitization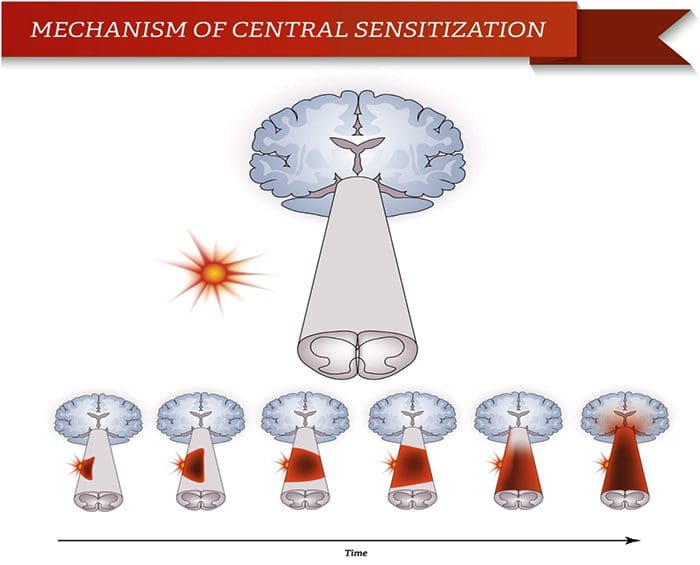
 Somatosensory Cortex Organization
Somatosensory Cortex Organization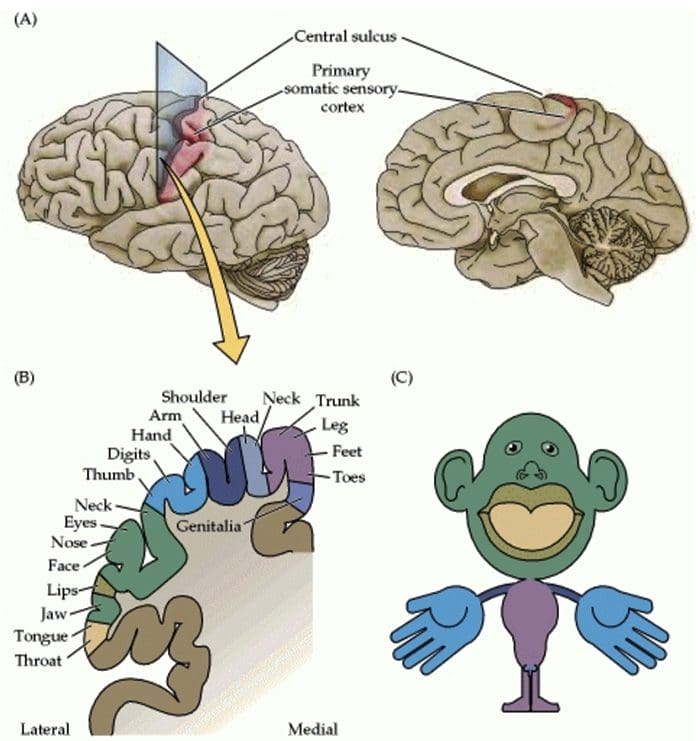 Cortical Reorganization
Cortical Reorganization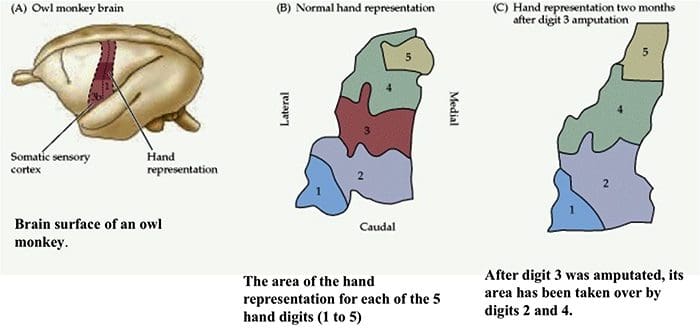
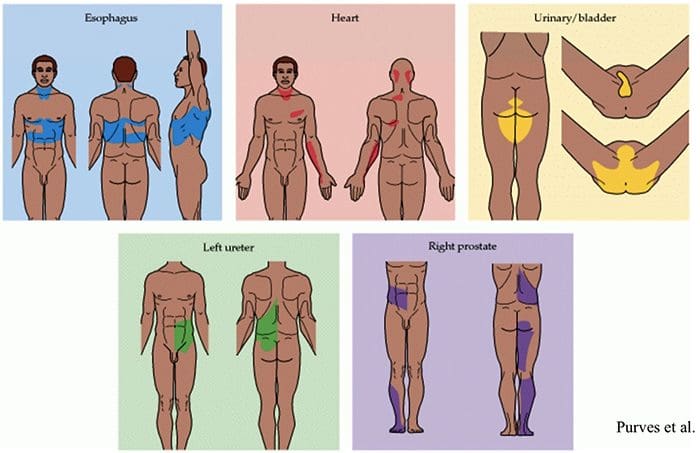
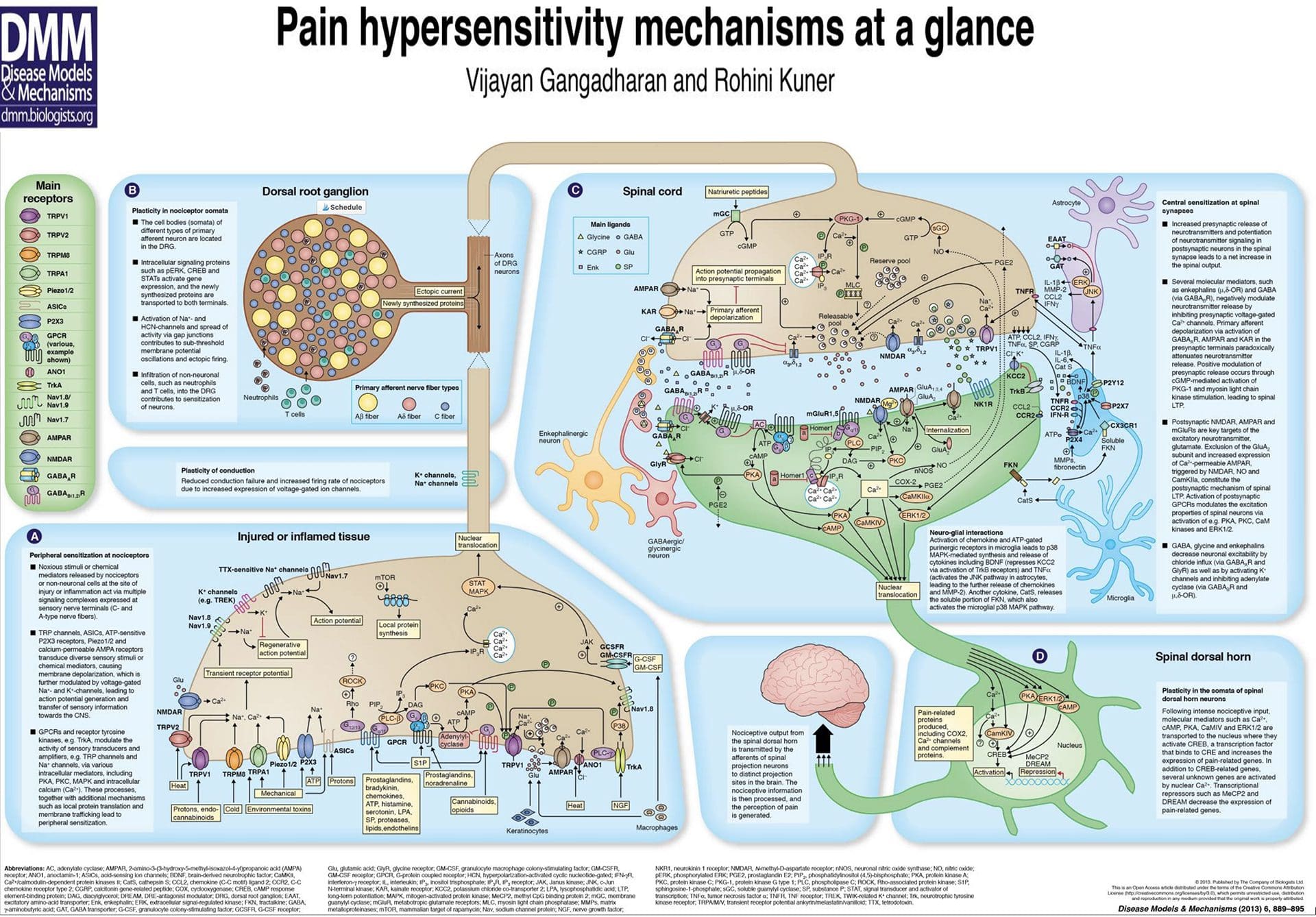

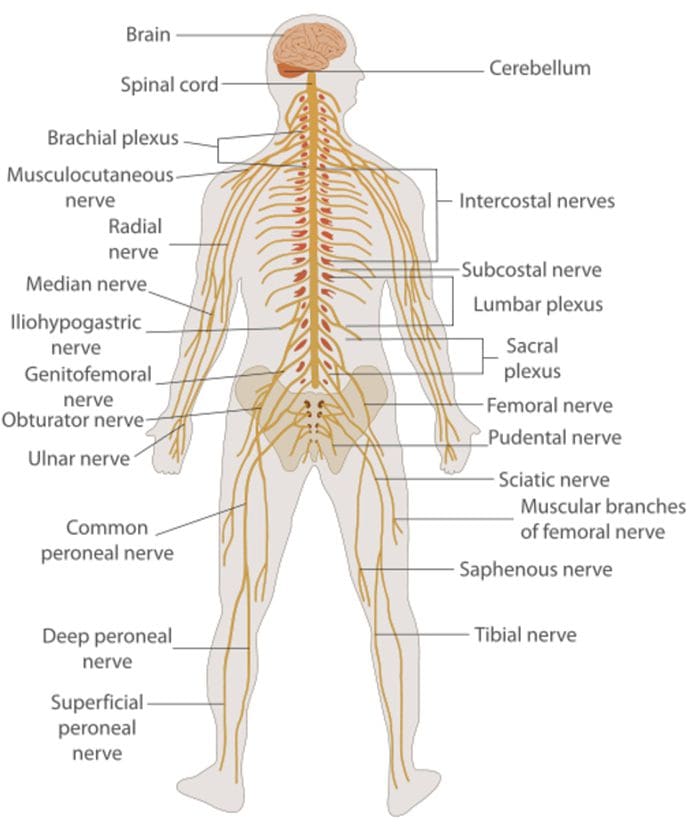
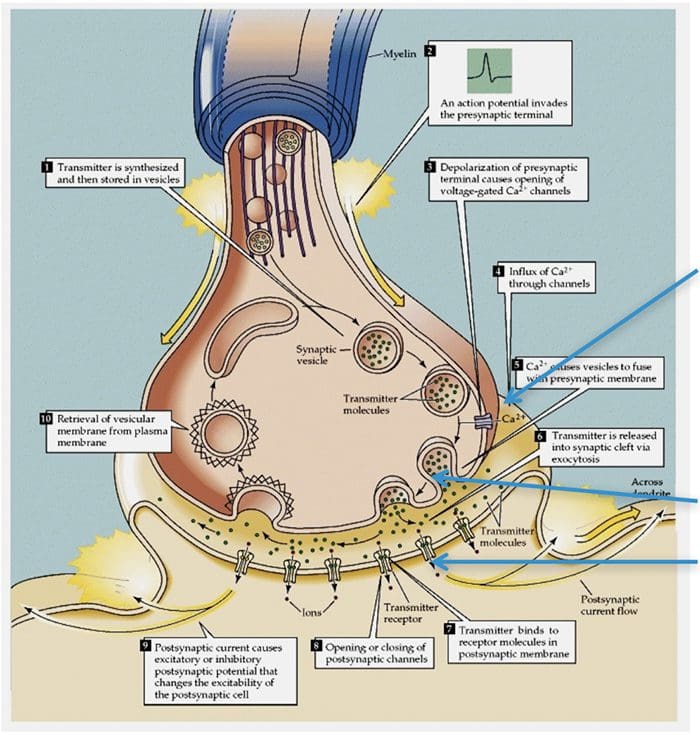
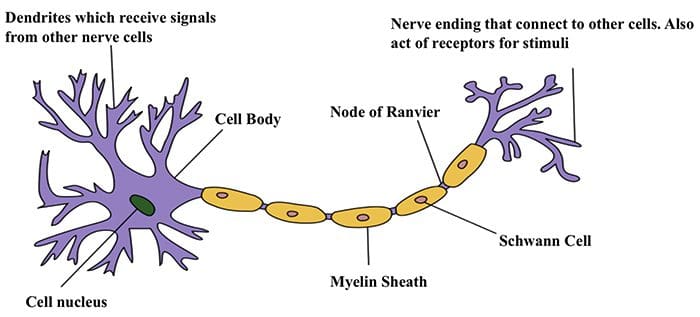 Nerve Cell-To-Nerve Cell Communication
Nerve Cell-To-Nerve Cell Communication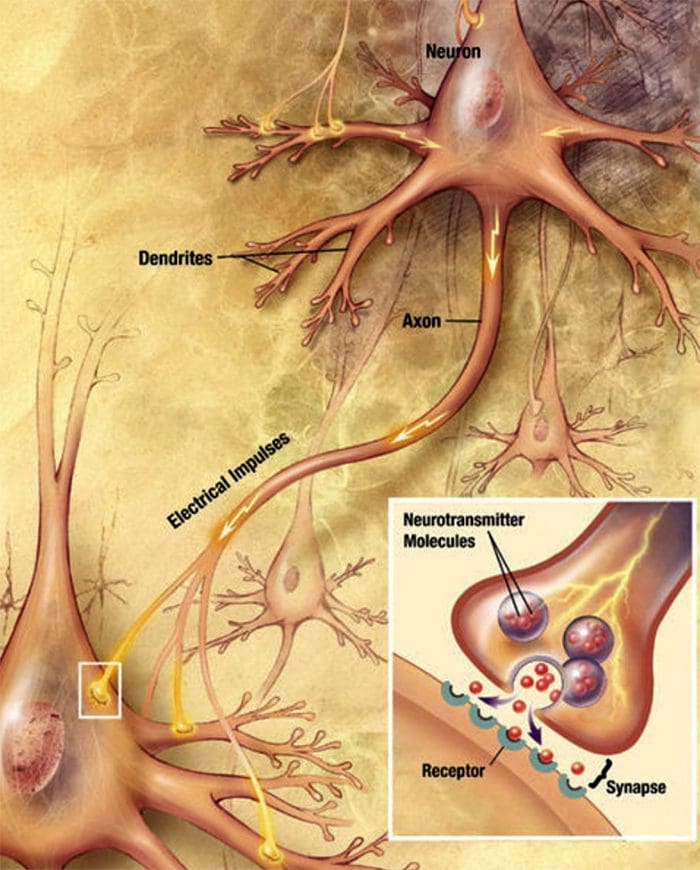 Nerve cells communicate with other cells by releasing a chemical from the nerve endings � Neurotransmitters
Nerve cells communicate with other cells by releasing a chemical from the nerve endings � Neurotransmitters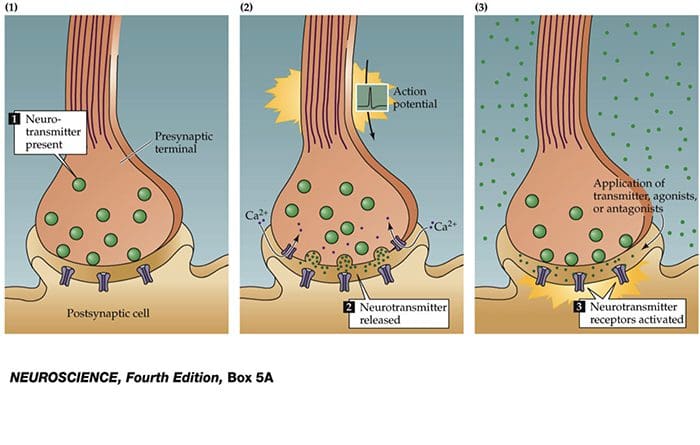
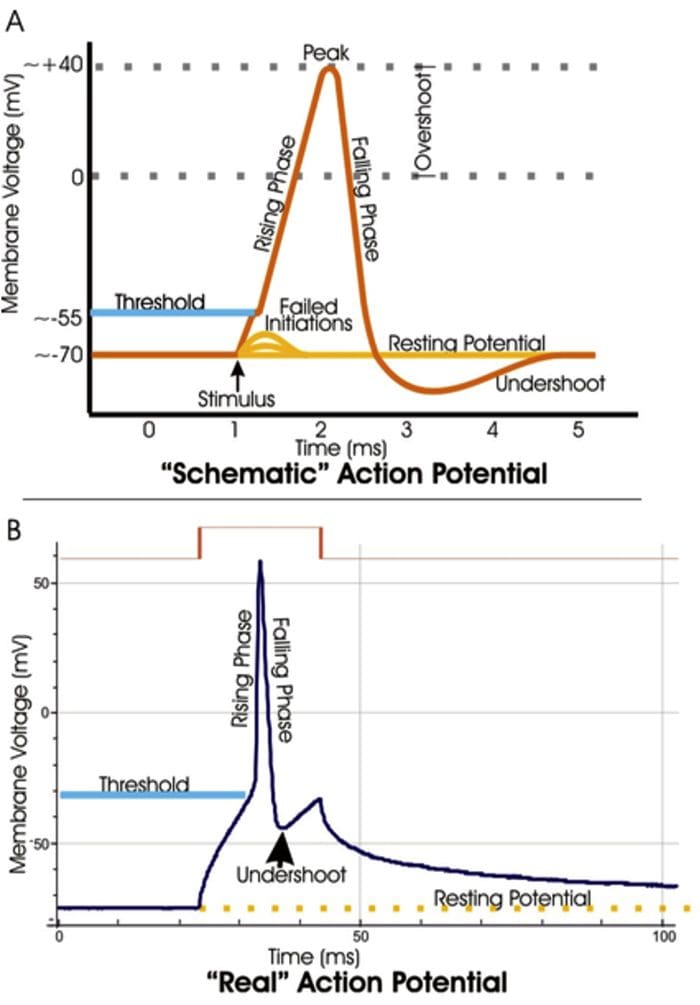
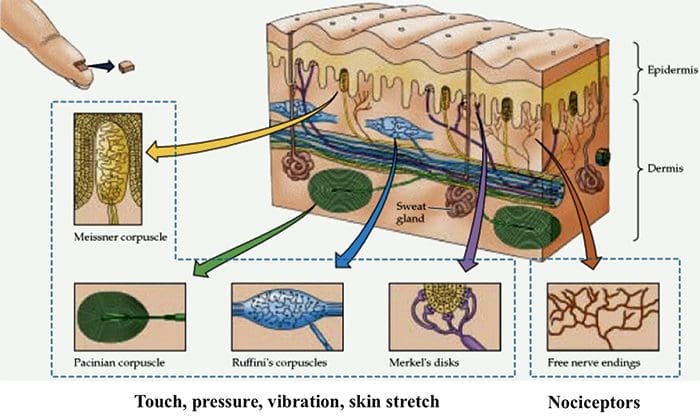
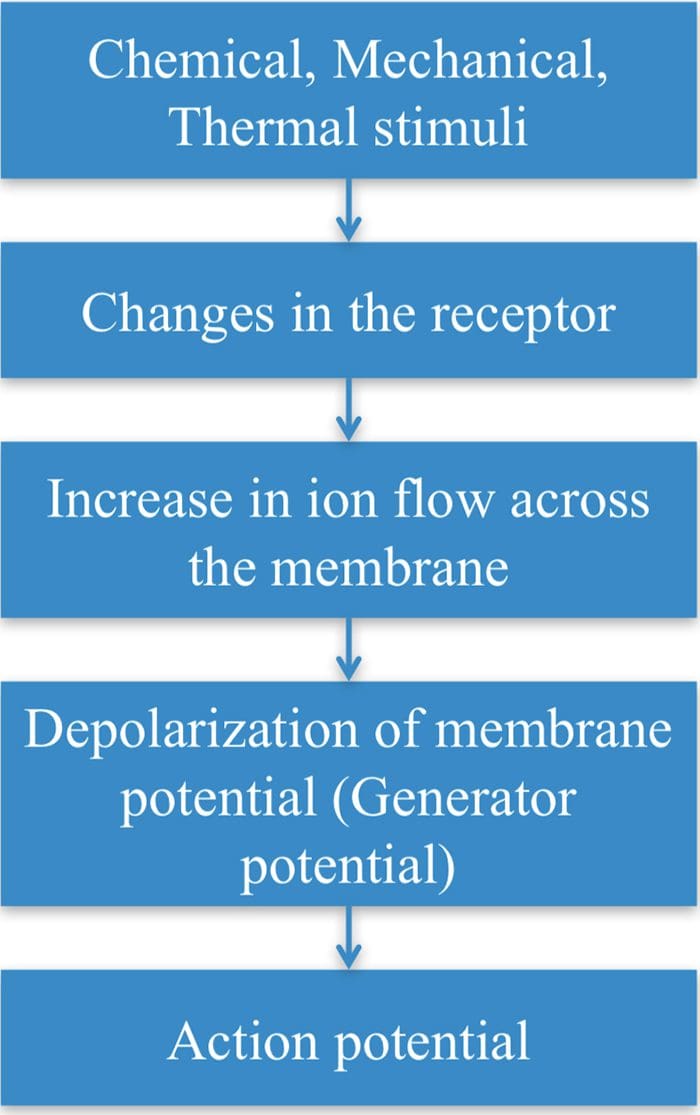 TRP Channels
TRP Channels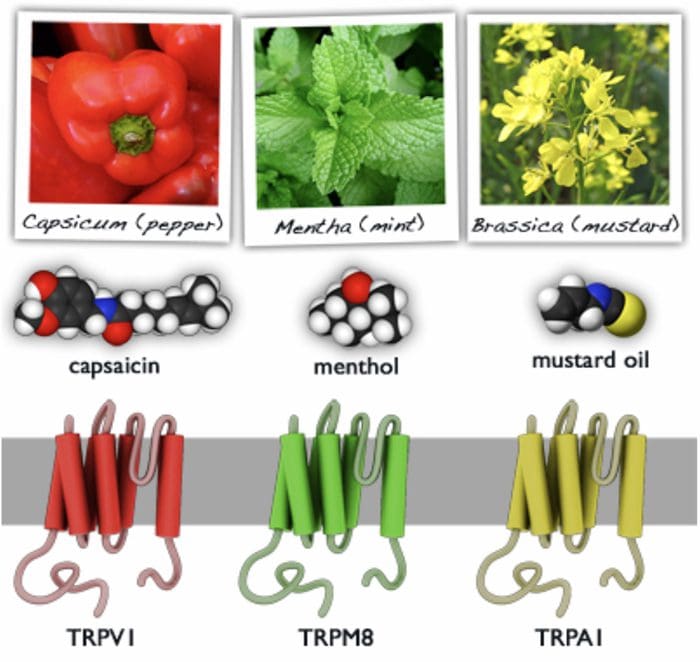



 We are blessed to present to you�El Paso�s Premier Wellness & Injury Care Clinic.
We are blessed to present to you�El Paso�s Premier Wellness & Injury Care Clinic.